Genes from mycoparasitic fungi as a source for improving plant resistance to fungal pathogens
- PMID: 9653105
- PMCID: PMC20894
- DOI: 10.1073/pnas.95.14.7860
Genes from mycoparasitic fungi as a source for improving plant resistance to fungal pathogens
Erratum in
- Proc Natl Acad Sci U S A 1998 Oct 13;95(21):12734. Fernandez IG [corrected to Garcia I]
Abstract
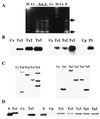
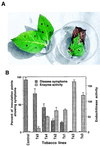
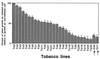
Disease resistance in transgenic plants has been improved, for the first time, by the insertion of a gene from a biocontrol fungus. The gene encoding a strongly antifungal endochitinase from the mycoparasitic fungus Trichoderma harzianum was transferred to tobacco and potato. High expression levels of the fungal gene were obtained in different plant tissues, which had no visible effect on plant growth and development. Substantial differences in endochitinase activity were detected among transformants. Selected transgenic lines were highly tolerant or completely resistant to the foliar pathogens Alternaria alternata, A. solani, Botrytis cinerea, and the soilborne pathogen Rhizoctonia solani. The high level and the broad spectrum of resistance obtained with a single chitinase gene from Trichoderma overcome the limited efficacy of transgenic expression in plants of chitinase genes from plants and bacteria. These results demonstrate a rich source of genes from biocontrol fungi that can be used to control diseases in plants.
Figures



Similar articles
-
Enhanced fungal resistance in transgenic cotton expressing an endochitinase gene from Trichoderma virens.Plant Biotechnol J. 2003 Sep;1(5):321-36. doi: 10.1046/j.1467-7652.2003.00029.x. Plant Biotechnol J. 2003. PMID: 17166131
-
Signal transduction by Tga3, a novel G protein alpha subunit of Trichoderma atroviride.Appl Environ Microbiol. 2005 Mar;71(3):1591-7. doi: 10.1128/AEM.71.3.1591-1597.2005. Appl Environ Microbiol. 2005. PMID: 15746364 Free PMC article.
-
Antioxidant genes of plants and fungal pathogens are distinctly regulated during disease development in different Rhizoctonia solani pathosystems.PLoS One. 2018 Feb 21;13(2):e0192682. doi: 10.1371/journal.pone.0192682. eCollection 2018. PLoS One. 2018. PMID: 29466404 Free PMC article.
-
[Lytic enzymes of Trichoderma and their role in protecting plants from fungal diseases].Prikl Biokhim Mikrobiol. 2003 Jul-Aug;39(4):389-400. Prikl Biokhim Mikrobiol. 2003. PMID: 14520957 Review. Russian.
-
[Perception of pathogen signals and gene-for-gene hypothesis].Tanpakushitsu Kakusan Koso. 1999 Nov;44(15 Suppl):2305-7. Tanpakushitsu Kakusan Koso. 1999. PMID: 10586673 Review. Japanese. No abstract available.
Cited by
-
Comparative genomics reveals a core gene toolbox for lifestyle transitions in Hypocreales fungi.Environ Microbiol. 2021 Jun;23(6):3251-3264. doi: 10.1111/1462-2920.15554. Epub 2021 May 11. Environ Microbiol. 2021. PMID: 33939870 Free PMC article.
-
Identification of differentially expressed genes from Trichoderma harzianum during growth on cell wall of Fusarium solani as a tool for biotechnological application.BMC Genomics. 2013 Mar 15;14:177. doi: 10.1186/1471-2164-14-177. BMC Genomics. 2013. PMID: 23497274 Free PMC article.
-
Mode of action and antifungal properties of two cold-adapted chitinases.Extremophiles. 2003 Oct;7(5):385-90. doi: 10.1007/s00792-003-0338-3. Epub 2003 Jul 15. Extremophiles. 2003. PMID: 12884086
-
Enhanced Tolerance against a Fungal Pathogen and Insect Resistance in Transgenic Tobacco Plants Overexpressing an Endochitinase Gene from Serratia marcescens.Int J Mol Sci. 2019 Jul 16;20(14):3482. doi: 10.3390/ijms20143482. Int J Mol Sci. 2019. PMID: 31315176 Free PMC article.
-
Genetically engineered Thompson Seedless grapevine plants designed for fungal tolerance: selection and characterization of the best performing individuals in a field trial.Transgenic Res. 2015 Feb;24(1):43-60. doi: 10.1007/s11248-014-9811-2. Epub 2014 Jul 11. Transgenic Res. 2015. PMID: 25011563
References
-
- Weindling R. Phytopathology. 1934;24:1153–1179.
-
- Harman G E, Hayes C K. Report to the Office of Technology Assessment. Washington, D.C.: U.S. Congress; 1996. pp. 34–96.
-
- Lorito M, Peterbauer C, Hayes C K, Harman G E. Microbiology. 1994;140:623–629. - PubMed
-
- Lorito M, Harman G E, Hayes C K, Broadway R M, Tronsmo A, Woo S L, Di Pietro A. Phytopathology. 1993;83:302–307.
-
- Lorito M, Hayes C K, Di Pietro A, Woo S L, Harman G E. Phytopathology. 1994;84:398–405.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

