The SbcCD nuclease of Escherichia coli is a structural maintenance of chromosomes (SMC) family protein that cleaves hairpin DNA
- PMID: 9653124
- PMCID: PMC20913
- DOI: 10.1073/pnas.95.14.7969
The SbcCD nuclease of Escherichia coli is a structural maintenance of chromosomes (SMC) family protein that cleaves hairpin DNA
Abstract
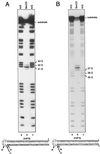
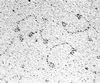
Hairpin structures can inhibit DNA replication and are intermediates in certain recombination reactions. We have shown that the purified SbcCD protein of Escherichia coli cleaves a DNA hairpin. This cleavage does not require the presence of a free (3' or 5') DNA end and generates products with 3'-hydroxyl and 5'-phosphate termini. Electron microscopy of SbcCD has revealed the "head-rod-tail" structure predicted for the SMC (structural maintenance of chromosomes) family of proteins, of which SbcC is a member. This work provides evidence consistent with the proposal that SbcCD cleaves hairpin structures that halt the progress of the replication fork, allowing homologous recombination to restore DNA replication.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

