Dioxygen activation and bond cleavage by mixed-valence cytochrome c oxidase
- PMID: 9653133
- PMCID: PMC20922
- DOI: 10.1073/pnas.95.14.8020
Dioxygen activation and bond cleavage by mixed-valence cytochrome c oxidase
Abstract
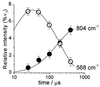
Elucidating the structures of intermediates in the reduction of O2 to water by cytochrome c oxidase is crucial to understanding both oxygen activation and proton pumping by the enzyme. In the work here, the reaction of O2 with the mixed-valence enzyme, in which only heme a3 and CuB in the binuclear center are reduced, has been followed by time-resolved resonance Raman spectroscopy. The results show that O==O bond cleavage occurs within the first 200 micros after reaction initiation; the presence of a uniquely stable Fe---O---O(H) peroxy species is not detected. The product of this rapid reaction is a heme a3 oxoferryl (FeIV==O) species, which requires that an electron donor in addition to heme a3 and CuB must be involved. The available evidence suggests that the additional donor is an amino acid side chain. Recent crystallographic data [Yoshikawa, S., Shinzawa-Itoh, K., Nakashima, R., Yaono, R., Yamashita, E., Inoue, N., Yao, M., Fei, M. J., Libeu, C. P., Mizushima, T., et al. Science, in press; Ostermeier, C., Harrenga, A. , Ermler, U. & Michel, H. (1997) Proc. Natl. Acad. Sci. USA 94, 10547-10553] show that one of the CuB ligands, His240, is cross-linked to Tyr244 and that this cross-linked tyrosyl is ideally positioned to participate in dioxygen activation. We propose a mechanism for O---O bond cleavage that proceeds by concerted hydrogen atom transfer from the cross-linked His---Tyr species to produce the product oxoferryl species, CuB2+---OH-, and the tyrosyl radical. This mechanism provides molecular structures for two key intermediates that drive the proton pump in oxidase; moreover, it has clear analogies to the proposed O---O bond forming chemistry that occurs during O2 evolution in photosynthesis.
Figures


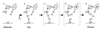
References
-
- Wikström M. Nature (London) 1977;266:271–273. - PubMed
-
- Babcock G T, Wikström M. Nature (London) 1992;356:301–309. - PubMed
-
- Ferguson-Miller S, Babcock G T. Chem Rev (Washington, D C) 1996;96:2889–2907. - PubMed
-
- Kitagawa T, Ogura T. In: Progress in Inorganic Chemistry. Karlin K D, editor. Vol. 45. New York: Wiley; 1997. pp. 431–479.
-
- Wikström M, Bogachev A, Finel M, Morgan J E, Puustinen A, Raitio M, Verkhovskaya M, Verkhovsky M I. Biochim Biophys Acta. 1994;1187:106–111. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

