Intrathymic selection of NK1.1(+)alpha/beta T cell antigen receptor (TCR)+ cells in transgenic mice bearing TCR specific for chicken ovalbumin and restricted to I-Ad
- PMID: 9653164
- PMCID: PMC20953
- DOI: 10.1073/pnas.95.14.8199
Intrathymic selection of NK1.1(+)alpha/beta T cell antigen receptor (TCR)+ cells in transgenic mice bearing TCR specific for chicken ovalbumin and restricted to I-Ad
Abstract
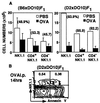
Generation and negative selection of NK1.1(+)alpha/beta T cell receptor (TCR)+ thymocytes were analyzed using TCR-transgenic (B10. D2 x DO10)F1 and (C57BL/6 x DO10)F1 mice and Rag-1(-/-)/DO10 mice, which had been established by breeding and backcrossing between Rag-1(-/-) and DO10 mice. Almost all T cells from these mice were shown to bear Valpha13/Vbeta8.2 that is specific for chicken ovalbumin (cOVA) and restricted to I-Ad. A normal proportion of the NK1.1(+) Valpha13/Vbeta8.2(+) thymocytes was generated in these mice. However, the actual cell number of both NK1.1(+) and NK1.1(-) thymocytes in I-Ad/d mice (positive selecting background) was larger than that in I-Ab/d mice (negative selecting background). Markedly low but significant proportions of NK1.1(+) Valpha13/Vbeta8.2(+) cells were detected in the spleens from I-Ad/d and I-Ab/d mice. It was shown that the splenic NK1.1(+) T cells of the I-Ab/d mice were anergized against stimulation through TCR. When (B10.D2 x DO10)F1 and (C57BL/6 x DO10)F1 mice were given cOVA, extensive or intermediate elimination of NK1.1(+)alpha/betaTCR+ thymocytes was induced in I-Ad/d or I-Ab/d mice, respectively. However, the clonal elimination was not as complete as that seen in the major NK1.1(-) thymocyte population. The present findings indicate that normal generation of NK1.1(+)alpha/betaTCR+ thymocytes occurs in the absence of Valpha14-Jalpha281 and that substantial negative selection operates on the NK1.1(+)alpha/betaTCR+ cells.
Figures





References
-
- Bendelac A. Curr Opin Immunol. 1995;7:367–374. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

