Nanobacteria: an alternative mechanism for pathogenic intra- and extracellular calcification and stone formation
- PMID: 9653177
- PMCID: PMC20966
- DOI: 10.1073/pnas.95.14.8274
Nanobacteria: an alternative mechanism for pathogenic intra- and extracellular calcification and stone formation
Abstract
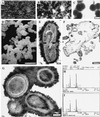
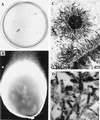
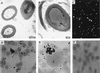
Calcium phosphate is deposited in many diseases, but formation mechanisms remain speculative. Nanobacteria are the smallest cell-walled bacteria, only recently discovered in human and cow blood and commercial cell culture serum. In this study, we identified with energy-dispersive x-ray microanalysis and chemical analysis that all growth phases of nanobacteria produce biogenic apatite on their cell envelope. Fourier transform IR spectroscopy revealed the mineral as carbonate apatite. The biomineralization in cell culture media resulted in biofilms and mineral aggregates closely resembling those found in tissue calcification and kidney stones. In nanobacteria-infected fibroblasts, electron microscopy revealed intra- and extracellular acicular crystal deposits, stainable with von Kossa staining and resembling calcospherules found in pathological calcification. Previous models for stone formation have led to an hypothesis that elevated pH due to urease and/or alkaline phosphatase activity is a lithogenic factor. Our results indicate that carbonate apatite can be formed without these factors at pH 7.4, at physiological phosphate and calcium concentrations. Nanobacteria can produce apatite in media mimicking tissue fluids and glomerular filtrate and provide a unique model for in vitro studies on calcification.
Figures

References
-
- Robey P G, Boskey A L. In: Osteoporosis. Marcus R, Feldman D, Kelsey J, editors. San Diego: Academic; 1996. pp. 95–183.
-
- Folk R L. J Sediment Petrol. 1993;63:990–999.
-
- Sillitoe R H, Folk R L, Saric N. Science (Washington DC) 1996;272:1153–1155. - PubMed
-
- Mojzsis S J, Arrhenius G, McKeegan K D, Harrison T M, Nutman A P, Friend C R L. Nature (London) 1996;384:55–59. - PubMed
-
- Çiftçioglu N, Kajander E O. Pathophysiology. 1998;4:259–270.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

