Different patterns of truncated prion protein fragments correlate with distinct phenotypes in P102L Gerstmann-Sträussler-Scheinker disease
- PMID: 9653185
- PMCID: PMC20974
- DOI: 10.1073/pnas.95.14.8322
Different patterns of truncated prion protein fragments correlate with distinct phenotypes in P102L Gerstmann-Sträussler-Scheinker disease
Abstract
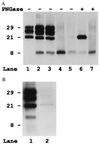
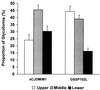
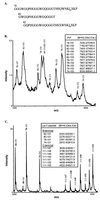
The clinicopathological phenotype of the Gerstmann-Sträussler-Scheinker disease (GSS) variant linked to the codon 102 mutation in the prion protein (PrP) gene (GSS P102L) shows a high heterogeneity. This variability also is observed in subjects with the same prion protein gene PRNP haplotype and is independent from the duration of the disease. Immunoblot analysis of brain homogenates from GSS P102L patients showed two major protease-resistant PrP fragments (PrP-res) with molecular masses of approximately 21 and 8 kDa, respectively. The 21-kDa fragment, similar to the PrP-res type 1 described in Creutzfeldt-Jakob disease, was found in five of the seven subjects and correlated with the presence of spongiform degeneration and "synaptic" pattern of PrP deposition whereas the 8-kDa fragment, similar to those described in other variants of GSS, was found in all subjects in brain regions showing PrP-positive multicentric amyloid deposits. These data further indicate that the neuropathology of prion diseases largely depends on the type of PrP-res fragment that forms in vivo. Because the formation of PrP-res fragments of 7-8 kDa with ragged N and C termini is not a feature of Creutzfeldt-Jakob disease or fatal familial insomnia but appears to be shared by most GSS subtypes, it may represent a molecular marker for this disorder.
Figures


References
-
- Parchi P, Piccardo P, Gambetti P, Ghetti B. In: Progress in Pathology 4. Kirkham N, Lemoine N R, editors. Edimburgh: Churchill Livingstone; 1998. pp. 39–77.
-
- Ghetti B, Piccardo P, Frangione B, Bugiani O, Giaccone G, Young K, Prelli F, Farlow M R, Dlouhy S R, Tagliavini F. Brain Pathol. 1996;6:127–145. - PubMed
-
- Parchi P, Castellani R, Capellari S, Ghetti B, Young K, Chen S G, Farlow M, Dickson D W, Sima A A F, Trojanowski J Q, et al. Ann Neurol. 1996;39:767–778. - PubMed
-
- Parchi P, Capellari S, Chen S G, Petersen R B, Gambetti P, Kopp N, Brown P, Kitamoto T, Tateishi J, Giese A, et al. Nature (London) 1997;386:232–234. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

