Proposed signal transduction role for conserved CheY residue Thr87, a member of the response regulator active-site quintet
- PMID: 9657998
- PMCID: PMC107323
- DOI: 10.1128/JB.180.14.3563-3569.1998
Proposed signal transduction role for conserved CheY residue Thr87, a member of the response regulator active-site quintet
Abstract
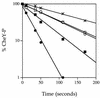
CheY serves as a structural prototype for the response regulator proteins of two-component regulatory systems. Functional roles have previously been defined for four of the five highly conserved residues that form the response regulator active site, the exception being the hydroxy amino acid which corresponds to Thr87 in CheY. To investigate the contribution of Thr87 to signaling, we characterized, genetically and biochemically, several cheY mutants with amino acid substitutions at this position. The hydroxyl group appears to be necessary for effective chemotaxis, as a Thr-->Ser substitution was the only one of six tested which retained a Che+ swarm phenotype. Although nonchemotactic, cheY mutants with amino acid substitutions T87A and T87C could generate clockwise flagellar rotation either in the absence of CheZ, a protein that stimulates dephosphorylation of CheY, or when paired with a second site-activating mutation, Asp13-->Lys, demonstrating that a hydroxy amino acid at position 87 is not essential for activation of the flagellar switch. All purified mutant proteins examined phosphorylated efficiently from the CheA kinase in vitro but were impaired in autodephosphorylation. Thus, the mutant CheY proteins are phosphorylated to a greater degree than wild-type CheY yet support less clockwise flagellar rotation. The data imply that Thr87 is important for generating and/or stabilizing the phosphorylation-induced conformational change in CheY. Furthermore, the various position 87 substitutions differentially affected several properties of the mutant proteins. The chemotaxis and autodephosphorylation defects were tightly linked, suggesting common structural elements, whereas the effects on self-catalyzed and CheZ-mediated dephosphorylation of CheY were uncorrelated, suggesting different structural requirements for the two dephosphorylation reactions.
Figures




References
-
- Appleby, J. L., and R. B. Bourret. Activation of CheY mutant D57N by phosphorylation at an alternate site, Ser56. Submitted for publication. - PubMed
-
- Barak R, Eisenbach M. Correlation between phosphorylation of the chemotaxis protein CheY and its activity at the flagellar motor. Biochemistry. 1992;31:1821–1826. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

