Circadian rhythm of nitrogenase gene expression in the diazotrophic filamentous nonheterocystous cyanobacterium Trichodesmium sp. strain IMS 101
- PMID: 9658003
- PMCID: PMC107328
- DOI: 10.1128/JB.180.14.3598-3605.1998
Circadian rhythm of nitrogenase gene expression in the diazotrophic filamentous nonheterocystous cyanobacterium Trichodesmium sp. strain IMS 101
Abstract
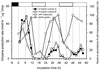
Recent studies suggested that the daily cycle of nitrogen fixation activity in the marine filamentous nonheterocystous cyanobacterium Trichodesmium sp. is controlled by a circadian rhythm. In this study, we evaluated the rhythm of nitrogen fixation in Trichodesmium sp. strain IMS 101 by using the three criteria for an endogenous rhythm. Nitrogenase transcript abundance oscillated with a period of approximately 24 h, and the cycle was maintained even under constant light conditions. The cyclic pattern of transcript abundance was maintained when the culture was grown at 24 and 28.5 degrees C, although the period was slightly longer (26 h) at the higher temperature. The cycle of gene expression could be entrained with light-dark cues. Results of inhibitor experiments indicated that transcript abundance was regulated primarily by transcription initiation, rather than by degradation. The circadian rhythm, the first conclusively demonstrated endogenous rhythm in a filamentous cyanobacterium, was also reflected in nitrogenase MoFe protein abundance and patterns of Fe protein posttranslational modification-demodification.
Figures









References
-
- Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K A. Current protocols in molecular biology. New York, N.Y: Greene Publishing and Wiley-Interscience; 1990.
-
- Bergman B B, Gallon J R, Rai A N, Stal L J. N2 fixation by non-heterocystous cyanobacteria. FEMS Microbiol Rev. 1997;19:139–185.
-
- Capone D G. Determination of nitrogenase activity in aquatic samples using the acetylene reduction procedure. In: Kemp P F, Sherr B F, Sherr E B, Cole J J, editors. Current methods in aquatic microbiology. New York, N.Y: Lewis Publishers, Inc.; 1993. pp. 621–631.
-
- Capone D G, Zehr J P, Paerl H W, Bergman B, Carpenter E J. Trichodesmium, a globally significant marine cyanobacterium. Science. 1997;276:1221–1229.
-
- Carpenter E J. Physiology and ecology of marine Oscillatoria (Trichodesmium) Mar Biol Lett. 1983;4:69–85.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

