Transfectant influenza A viruses with long deletions in the NS1 protein grow efficiently in Vero cells
- PMID: 9658085
- PMCID: PMC109801
- DOI: 10.1128/JVI.72.8.6437-6441.1998
Transfectant influenza A viruses with long deletions in the NS1 protein grow efficiently in Vero cells
Abstract
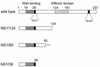
We established a reverse genetics system for the nonstructural (NS) gene segment of influenza A virus. This system is based on the use of the temperature-sensitive (ts) reassortant virus 25A-1. The 25A-1 virus contains the NS gene from influenza A/Leningrad/134/57 virus and the remaining gene segments from A/Puerto Rico (PR)/8/34 virus. This particular gene constellation was found to be responsible for the ts phenotype. For reverse genetics of the NS gene, a plasmid-derived NS gene from influenza A/PR/8/34 virus was ribonucleoprotein transfected into cells that were previously infected with the 25A-1 virus. Two subsequent passages of the transfection supernatant at 40 degreesC selected viruses containing the transfected NS gene derived from A/PR/8/34 virus. The high efficiency of the selection process permitted the rescue of transfectant viruses with large deletions of the C-terminal part of the NS1 protein. Viable transfectant viruses containing the N-terminal 124, 80, or 38 amino acids of the NS1 protein were obtained. Whereas all deletion mutants grew to high titers in Vero cells, growth on Madin-Darby canine kidney (MDCK) cells and replication in mice decreased with increasing length of the deletions. In Vero cells expression levels of viral proteins of the deletion mutants were similar to those of the wild type. In contrast, in MDCK cells the level of the M1 protein was significantly reduced for the deletion mutants.
Figures
References
-
- Egorov A, Garmashova L M, Lukashok I V, Nevedomskaia G N, Aleksandrova G I, Klimov A I. The NS gene—a possible determinant of apathogenicity of a cold-adapted donor of attenuation A/Leningrad/134/47/57 and its reassortants. Vopr Virusol. 1994;39:201–205. . (In Russian.) - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

