Involvement of aminopeptidase N (CD13) in infection of human neural cells by human coronavirus 229E
- PMID: 9658094
- PMCID: PMC109818
- DOI: 10.1128/JVI.72.8.6511-6519.1998
Involvement of aminopeptidase N (CD13) in infection of human neural cells by human coronavirus 229E
Abstract
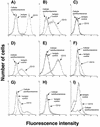
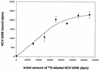
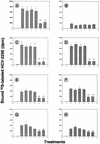
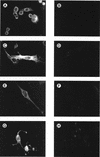
Attachment to a cell surface receptor can be a major determinant of virus tropism. Previous studies have shown that human respiratory coronavirus HCV-229E uses human aminopeptidase N (hAPN [CD13]) as its cellular receptor for infection of lung fibroblasts. Although human coronaviruses are recognized respiratory pathogens, occasional reports have suggested their possible neurotropism. We have previously shown that human neural cells, including glial cells in primary cultures, are susceptible to human coronavirus infection in vitro (A. Bonavia, N. Arbour, V. W. Yong, and P. J. Talbot, J. Virol. 71:800-806, 1997). However, the only reported expression of hAPN in the nervous system is at the level of nerve synapses. Therefore, we asked whether hAPN is utilized as a cellular receptor for infection of these human neural cell lines. Using flow cytometry, we were able to show the expression of hAPN on the surfaces of various human neuronal and glial cell lines that are susceptible to HCV-229E infection. An hAPN-specific monoclonal antibody (WM15), but not control antibody, inhibited the attachment of radiolabeled HCV-229E to astrocytic, neuronal, and oligodendrocytic cell lines. A correlation between the apparent amount of cell surface hAPN and the level of virus attachment was observed. Furthermore, the presence of WM15 inhibited virus infection of these cell lines, as detected by indirect immunofluorescence. These results indicate that hAPN (CD13) is expressed on neuronal and glial cell lines in vitro and serves as the receptor for infection by HCV-229E. This further strengthens the neurotropic potential of this human respiratory virus.
Figures

References
-
- Arbour N, Talbot P J. Persistent infection of neural cell lines by human coronaviruses. Adv Exp Med Biol. 1998;440:575–581. - PubMed
-
- Arbour, N., and P. J. Talbot. 1998. Unpublished data.
-
- Ashmun R A, Look A T. Metalloprotease activity of CD13/aminopeptidase N on the surface of human myeloid cells. Blood. 1990;75:462–469. - PubMed
-
- Barnes K, Kenny A J, Turner A J. Localization of aminopeptidase N and dipeptidyl peptidase IV in pig striatum and in neuronal and glial cell cultures. Eur J Neurosci. 1994;6:531–537. - PubMed
-
- Barnett E M, Perlman S. The olfactory nerve and not the trigeminal nerve is the major site of CNS entry for mouse hepatitis virus, strain JHM. Virology. 1993;194:185–191. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

