The disruption of ND10 during herpes simplex virus infection correlates with the Vmw110- and proteasome-dependent loss of several PML isoforms
- PMID: 9658103
- PMCID: PMC109835
- DOI: 10.1128/JVI.72.8.6581-6591.1998
The disruption of ND10 during herpes simplex virus infection correlates with the Vmw110- and proteasome-dependent loss of several PML isoforms
Abstract
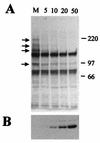
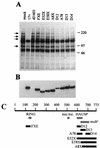
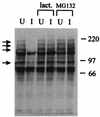
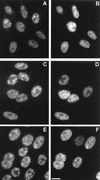
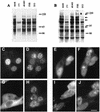
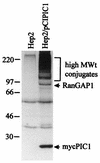
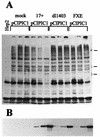
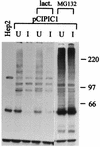
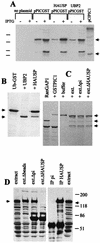
The small nuclear structures known as ND10 or PML nuclear bodies have been implicated in a variety of cellular processes including response to stress and interferons, oncogenesis, and viral infection, but little is known about their biochemical properties. Recently, a ubiquitin-specific protease enzyme (named HAUSP) and a ubiquitin-homology family protein (PIC1) have been found associated with ND10. HAUSP binds strongly to Vmw110, a herpesvirus regulatory protein which has the ability to disrupt ND10, while PIC1 was identified as a protein which interacts with PML, the prototype ND10 protein. We have investigated the role of ubiquitin-related pathways in the mechanism of ND10 disruption by Vmw110 and the effect of virus infection on PML stability. The results show that the disruption of ND10 during virus infection correlates with the loss of several PML isoforms and this process is dependent on active proteasomes. The PML isoforms that are most sensitive to virus infection correspond closely to those which have recently been identified as being covalently conjugated to PIC1. In addition, a large number of PIC1-protein conjugates can be detected following transfection of a PIC1 expression plasmid, and many of these are also eliminated in a Vmw110-dependent manner during virus infection. These observations provide a biochemical mechanism to explain the observed effects of Vmw110 on ND10 and suggest a simple yet powerful mechanism by which Vmw110 might function during virus infection.
Figures
Similar articles
-
Alphaherpesvirus proteins related to herpes simplex virus type 1 ICP0 affect cellular structures and proteins.J Virol. 2000 Nov;74(21):10006-17. doi: 10.1128/jvi.74.21.10006-10017.2000. J Virol. 2000. PMID: 11024129 Free PMC article.
-
A novel ubiquitin-specific protease is dynamically associated with the PML nuclear domain and binds to a herpesvirus regulatory protein.EMBO J. 1997 Apr 1;16(7):1519-30. doi: 10.1093/emboj/16.7.1519. EMBO J. 1997. PMID: 9130697 Free PMC article.
-
A novel ubiquitin-specific protease is dynamically associated with the PML nuclear domain and binds to a herpesvirus regulatory protein.EMBO J. 1997 Feb 3;16(3):566-77. doi: 10.1093/emboj/16.3.566. EMBO J. 1997. Corrected and republished in: EMBO J. 1997 Apr 1;16(7):1519-30. doi: 10.1093/emboj/16.7.1519. PMID: 9034339 Free PMC article. Corrected and republished.
-
PML and COP1--two proteins with much in common.Trends Biochem Sci. 2001 Jan;26(1):18-20. doi: 10.1016/s0968-0004(00)01732-1. Trends Biochem Sci. 2001. PMID: 11165511 Review.
-
The use of fluorescence microscopy to study the association between herpesviruses and intrinsic resistance factors.Viruses. 2011 Dec;3(12):2412-24. doi: 10.3390/v3122412. Epub 2011 Dec 7. Viruses. 2011. PMID: 22355446 Free PMC article. Review.
Cited by
-
Differential Roles of PML Isoforms.Front Oncol. 2013 May 22;3:125. doi: 10.3389/fonc.2013.00125. eCollection 2013. Front Oncol. 2013. PMID: 23734343 Free PMC article.
-
Role of ND10 nuclear bodies in the chromatin repression of HSV-1.Virol J. 2016 Apr 5;13:62. doi: 10.1186/s12985-016-0516-4. Virol J. 2016. PMID: 27048561 Free PMC article. Review.
-
Functions of the Epstein-Barr virus EBNA1 protein in viral reactivation and lytic infection.J Virol. 2012 Jun;86(11):6146-58. doi: 10.1128/JVI.00013-12. Epub 2012 Apr 4. J Virol. 2012. PMID: 22491455 Free PMC article.
-
Crystal Structure of USP7 Ubiquitin-like Domains with an ICP0 Peptide Reveals a Novel Mechanism Used by Viral and Cellular Proteins to Target USP7.PLoS Pathog. 2015 Jun 5;11(6):e1004950. doi: 10.1371/journal.ppat.1004950. eCollection 2015 Jun. PLoS Pathog. 2015. PMID: 26046769 Free PMC article.
-
Tegument Proteins of Kaposi's Sarcoma-Associated Herpesvirus and Related Gamma-Herpesviruses.Front Microbiol. 2012 Mar 15;3:98. doi: 10.3389/fmicb.2012.00098. eCollection 2012. Front Microbiol. 2012. PMID: 22435068 Free PMC article.
References
-
- Boddy M N, Howe K, Etkin L D, Solomon E, Freemont P S. PIC1, a novel ubiquitin-like protein which interacts with the PML component of a multiprotein complex that is disrupted in acute promyelocytic leukaemia. Oncogene. 1996;13:971–982. - PubMed
-
- Boddy M N, Duprez E, Borden K L B, Freemont P S. Surface residue mutations of the PML RING finger domain alter the formation of nuclear-matrix-associated PML bodies. J Cell Sci. 1997;110:2197–2205. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

