Protective role of gamma interferon during the recall response to influenza virus
- PMID: 9658110
- PMCID: PMC109853
- DOI: 10.1128/JVI.72.8.6637-6645.1998
Protective role of gamma interferon during the recall response to influenza virus
Abstract
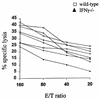
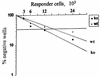
During secondary immune responses to influenza virus, virus-specific T memory cells are a major source of gamma interferon (IFN-gamma). We assessed the contribution of IFN-gamma to heterologous protection against the A/WSN/33 (H1N1) virus of wild-type and IFN-gamma-/- mice previously immunized with the A/HK/68 (H3N2) virus. The IFN-gamma-/- mice displayed significantly reduced survival rates subsequent to a challenge with various doses of the A/WSN/33 virus. This was associated with an impaired ability of the IFN-gamma-/- mice to completely clear the pulmonary virus by day 7 after the challenge, although significant reduction of the virus titers was noted. However, the IFN-gamma-/- mice developed type A influenza virus cross-reactive cytotoxic T lymphocytes (CTLs) similar to the wild-type mice, as demonstrated by both cytotoxicity and a limiting-dilution assay for the estimation of CTL precursor frequency. The pulmonary recruitment of T cells in IFN-gamma-/- mice was not dramatically affected, and the percentage of CD4(+) and CD8(+) T cells was similar to that of wild-type mice. The T cells from IFN-gamma-/- mice did not display a significant switch toward a Th2 profile. Furthermore, the IFN-gamma-/- mice retained the ability to mount significant titers of WSN and HK virus-specific hemagglutination-inhibiting antibodies. Together, these results are consistent with a protective role of IFN-gamma during the heterologous response against influenza virus independently of the generation and local recruitment of cross-reactive CTLs.
Figures

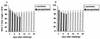
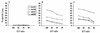

References
-
- Allan W, Tabi Z, Clearly A, Doherty P C. Cellular events in the lymph node and lung of mice infected with influenza. Consequences of depleting CD4+ T cells. J Immunol. 1990;144:3980–3986. - PubMed
-
- Billiau A. Interferon-γ: biology and role in pathogenesis. Adv Immunol. 1996;62:61–130. - PubMed
-
- Bot A, Bot S, Garcia-Sastre A, Bona C. DNA immunization of newborn mice with a plasmid expressing nucleoprotein of influenza virus. Viral Immunol. 1996;9:207–210. - PubMed
-
- Bot A, Casares S, Bot S, von Boehmer H, Bona C. Cellular mechanisms involved in protection against influenza virus infection of transgenic mice expressing a TCR receptor specific for class II-hemagglutinin peptide in CD4+ and CD8+ T cells. J Immunol. 1998;160:4500–4507. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

