Comparisons of highly virulent H5N1 influenza A viruses isolated from humans and chickens from Hong Kong
- PMID: 9658115
- PMCID: PMC109865
- DOI: 10.1128/JVI.72.8.6678-6688.1998
Comparisons of highly virulent H5N1 influenza A viruses isolated from humans and chickens from Hong Kong
Abstract
Genes of an influenza A (H5N1) virus from a human in Hong Kong isolated in May 1997 were sequenced and found to be all avian-like (K. Subbarao et al., Science 279:393-395, 1998). Gene sequences of this human isolate were compared to those of a highly pathogenic chicken H5N1 influenza virus isolated from Hong Kong in April 1997. Sequence comparisons of all eight RNA segments from the two viruses show greater than 99% sequence identity between them. However, neither isolate's gene sequence was closely (>95% sequence identity) related to any other gene sequences found in the GenBank database. Phylogenetic analysis demonstrated that the nucleotide sequences of at least four of the eight RNA segments clustered with Eurasian origin avian influenza viruses. The hemagglutinin gene phylogenetic analysis also included the sequences from an additional three human and two chicken H5N1 virus isolates from Hong Kong, and the isolates separated into two closely related groups. However, no single amino acid change separated the chicken origin and human origin isolates, but they all contained multiple basic amino acids at the hemagglutinin cleavage site, which is associated with a highly pathogenic phenotype in poultry. In experimental intravenous inoculation studies with chickens, all seven viruses were highly pathogenic, killing most birds within 24 h. All infected chickens had virtually identical pathologic lesions, including moderate to severe diffuse edema and interstitial pneumonitis. Viral nucleoprotein was most frequently demonstrated in vascular endothelium, macrophages, heterophils, and cardiac myocytes. Asphyxiation from pulmonary edema and generalized cardiovascular collapse were the most likely pathogenic mechanisms responsible for illness and death. In summary, a small number of changes in hemagglutinin gene sequences defined two closely related subgroups, with both subgroups having human and chicken members, among the seven viruses examined from Hong Kong, and all seven viruses were highly pathogenic in chickens and caused similar lesions in experimental inoculations.
Figures




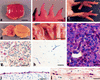
 ) (bar = 2.5 nm). (c) Normal blood
capillary endothelium (E) and air capillary epithelium (A) without
inflammatory cells (bar = 2 nm). (d) Thin basal lamina
(arrowheads) separating blood capillary endothelium (E) and air
capillary epithelium (A) (bar = 2.5 nm).
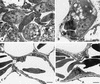
) (bar = 2.5 nm). (c) Normal blood
capillary endothelium (E) and air capillary epithelium (A) without
inflammatory cells (bar = 2 nm). (d) Thin basal lamina
(arrowheads) separating blood capillary endothelium (E) and air
capillary epithelium (A) (bar = 2.5 nm).References
-
- Alexander D J. Avian influenza. Recent developments. Vet Bull. 1982;52:341–359.
-
- Barbeito M S, Abraham G, Best M, Cairns P, Langevin P, Sterritt W G, Barr D, Meulepas W, Sanchez-Vizcaino J M, Saraza M, Requena E, Collado M, Mani P, Breeze R, Brunner H, Mebus C A, Morgan R L, Rusk S, Siegfried L M, Thompson L H. Recommended biocontainment features for research and diagnostic facilities where animal pathogens are used. Rev Sci Tech Off Int Epizoot. 1995;14:873–887. - PubMed
-
- Bashiruddin J B, Gould A R, Westbury H A. Molecular pathotyping of two avian influenza viruses isolated during the Victoria 1976 outbreak. Aust Vet J. 1992;69:140–142. - PubMed
-
- Beare A S, Webster R G. Replication of avian influenza viruses in humans. Arch Virol. 1991;119:37–42. - PubMed
-
- Brown C C, Olander H J, Senne D A. A pathogenesis study of highly pathogenic avian influenza virus H5N2 in chickens, using immunohistochemistry. J Comp Pathol. 1992;107:341–348. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

