The high mobility group protein 1 is a coactivator of herpes simplex virus ICP4 in vitro
- PMID: 9658123
- PMCID: PMC109883
- DOI: 10.1128/JVI.72.8.6752-6757.1998
The high mobility group protein 1 is a coactivator of herpes simplex virus ICP4 in vitro
Abstract
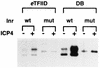
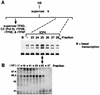
ICP4 is an activator of herpes simplex virus early and late gene transcription during infection and in vitro can efficiently activate the transcription of a core promoter template containing only a TATA box and an initiator element. In this study, we noted that the extent of activation by ICP4 in vitro was highly dependent on the purity of TFIID when recombinant TFIIB, TFIIE, and TFIIF were used as sources of these factors. ICP4 efficiently activated transcription with a crude TFIID fraction. However, when immunoaffinity-purified TFIID was used in place of the less pure TFIID, ICP4 activated transcription to a significantly lesser extent. This finding indicated that the crude TFIID fraction may contain additional factors that serve as coactivators of ICP4. To test this hypothesis, the crude TFIID preparation was further fractionated by gel filtration chromatography. The TFIID that eluted from the column lacked the hypothesized coactivator activity. A fraction well separated from TFIID contained an activity that when added with the TFIID fraction resulted in higher levels of transcription in the presence ICP4. Further purification of the coactivator-containing fraction resulted in the isolation of a single 30-kDa polypeptide (p30). p30 was also shown to serve as a coactivator of ICP4 with immunoaffinity-purified TFIID; however, p30 had no effect on basal transcription. Amino acid sequence analysis revealed that p30 was the high mobility group protein 1, which has been shown to facilitate the formation of higher-order DNA-protein complexes.
Figures
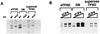



References
-
- Alwine J, Steinhart W L, Hill C W. Transcription of herpes simplex type 1 DNA in nuclei isolated from infected Hep-2 and KB cells. Virology. 1974;60:302–307. - PubMed
-
- Chen J-L, Donatella Attardi L, Verrijzer C P, Yokomori K, Tjian R. Assembly of recombinant TFIID reveals differential coactivator requirements for distinct transcriptional activators. Cell. 1994;79:93–105. - PubMed
-
- Coen D M, Weinheimer S P, McKnight S L. A genetic approach to promoter recognition during trans induction of viral gene expression. Science. 1986;234:53–59. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

