Localization of autocrine motility factor receptor to caveolae and clathrin-independent internalization of its ligand to smooth endoplasmic reticulum
- PMID: 9658170
- PMCID: PMC25416
- DOI: 10.1091/mbc.9.7.1773
Localization of autocrine motility factor receptor to caveolae and clathrin-independent internalization of its ligand to smooth endoplasmic reticulum
Abstract
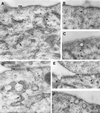
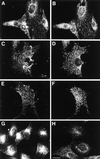
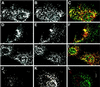
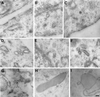
Autocrine motility factor receptor (AMF-R) is a cell surface receptor that is also localized to a smooth subdomain of the endoplasmic reticulum, the AMF-R tubule. By postembedding immunoelectron microscopy, AMF-R concentrates within smooth plasmalemmal vesicles or caveolae in both NIH-3T3 fibroblasts and HeLa cells. By confocal microscopy, cell surface AMF-R labeled by the addition of anti-AMF-R antibody to viable cells at 4 degreesC exhibits partial colocalization with caveolin, confirming the localization of cell surface AMF-R to caveolae. Labeling of cell surface AMF-R by either anti-AMF-R antibody or biotinylated AMF (bAMF) exhibits extensive colocalization and after a pulse of 1-2 h at 37 degreesC, bAMF accumulates in densely labeled perinuclear structures as well as fainter tubular structures that colocalize with AMF-R tubules. After a subsequent 2- to 4-h chase, bAMF is localized predominantly to AMF-R tubules. Cytoplasmic acidification, blocking clathrin-mediated endocytosis, results in the essentially exclusive distribution of internalized bAMF to AMF-R tubules. By confocal microscopy, the tubular structures labeled by internalized bAMF show complete colocalization with AMF-R tubules. bAMF internalized in the presence of a 10-fold excess of unlabeled AMF labels perinuclear punctate structures, which are therefore the product of fluid phase endocytosis, but does not label AMF-R tubules, demonstrating that bAMF targeting to AMF-R tubules occurs via a receptor-mediated pathway. By electron microscopy, bAMF internalized for 10 min is located to cell surface caveolae and after 30 min is present within smooth and rough endoplasmic reticulum tubules. AMF-R is therefore internalized via a receptor-mediated clathrin-independent pathway to smooth ER. The steady state localization of AMF-R to caveolae implicates these cell surface invaginations in AMF-R endocytosis.
Figures


References
-
- Anderson RGW, Kamen BA, Rothberg KG, Lacey SW. Potocytosis: sequestration and transport of small molecules by caveolae. Science. 1992;253:410–411. - PubMed
-
- Bendayan M, Rasio EA. Transport of insulin and albumin by the microvascular endothelium of the rete mirabile. J Cell Sci. 1996;109:1857–1864. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

