Multiple functions for actin during filamentous growth of Saccharomyces cerevisiae
- PMID: 9658177
- PMCID: PMC25429
- DOI: 10.1091/mbc.9.7.1873
Multiple functions for actin during filamentous growth of Saccharomyces cerevisiae
Abstract
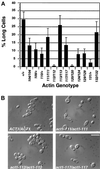
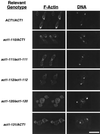
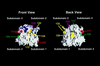
Saccharomyces cerevisiae is dimorphic and switches from a yeast form to a pseudohyphal (PH) form when starved for nitrogen. PH cells are elongated, bud in a unipolar manner, and invade the agar substrate. We assessed the requirements for actin in mediating the dramatic morphogenetic events that accompany the transition to PH growth. Twelve "alanine scan" alleles of the single yeast actin gene (ACT1) were tested for effects on filamentation, unipolar budding, agar invasion, and cell elongation. Some act1 mutations affect all phenotypes, whereas others affect only one or two aspects of PH growth. Tests of intragenic complementation among specific act1 mutations support the phenotypic evidence for multiple actin functions in filamentous growth. We present evidence that interaction between actin and the actin-binding protein fimbrin is important for PH growth and suggest that association of different actin-binding proteins with actin mediates the multiple functions of actin in filamentous growth. Furthermore, characterization of cytoskeletal structure in wild type and act1/act1 mutants indicates that PH cell morphogenesis requires the maintenance of a highly polarized actin cytoskeleton. Collectively, this work demonstrates that actin plays a central role in fungal dimorphism.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

