RhoA GTPase and serum response factor control selectively the expression of MyoD without affecting Myf5 in mouse myoblasts
- PMID: 9658178
- PMCID: PMC25431
- DOI: 10.1091/mbc.9.7.1891
RhoA GTPase and serum response factor control selectively the expression of MyoD without affecting Myf5 in mouse myoblasts
Abstract
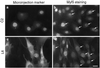
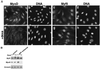
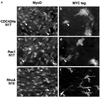
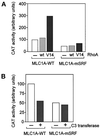
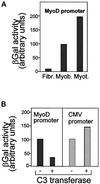
MyoD and Myf5 belong to the family of basic helix-loop-helix transcription factors that are key operators in skeletal muscle differentiation. MyoD and Myf5 genes are selectively activated during development in a time and region-specific manner and in response to different stimuli. However, molecules that specifically regulate the expression of these two genes and the pathways involved remain to be determined. We have recently shown that the serum response factor (SRF), a transcription factor involved in activation of both mitogenic response and muscle differentiation, is required for MyoD gene expression. We have investigated here whether SRF is also involved in the control of Myf5 gene expression, and the potential role of upstream regulators of SRF activity, the Rho family G-proteins including Rho, Rac, and CDC42, in the regulation of MyoD and Myf5. We show that inactivation of SRF does not alter Myf5 gene expression, whereas it causes a rapid extinction of MyoD gene expression. Furthermore, we show that RhoA, but not Rac or CDC42, is also required for the expression of MyoD. Indeed, blocking the activity of G-proteins using the general inhibitor lovastatin, or more specific antagonists of Rho proteins such as C3-transferase or dominant negative RhoA protein, resulted in a dramatic decrease of MyoD protein levels and promoter activity without any effects on Myf5 expression. We further show that RhoA-dependent transcriptional activation required functional SRF in C2 muscle cells. These data illustrate that MyoD and Myf5 are regulated by different upstream activation pathways in which MyoD expression is specifically modulated by a RhoA/SRF signaling cascade. In addition, our results establish the first link between RhoA protein activity and the expression of a key muscle regulator.
Figures



References
-
- Afti A, Djelloul S, Chastre E, Davis R, Gespach C. Evidence for a role of Rho-like GTPases and stress-activated protein kinase/c-jun N-terminal kinase (SAPK/JNK) in transforming Growth factor β-mediated signalling. J Biol Chem. 1997;272:1429–1432. - PubMed
-
- Aktories K, Hall A. Botulinum ADP-ribosyl transferase: a new tool to study low molecular weight GTP-binding proteins. Trends Pharmacol Sci. 1989;10:415–418. - PubMed
-
- Alberts AS, Geneste O, Treisman R. Activation of SRF-regulated chromosomal templates by Rho-family GTPases requires a signal that also induces H4 hyperacetylation. Cell. 1998;92:475–487. - PubMed
-
- Auradé F, Pfarr CM, Lindon C, Garcia A, Primig M, Montarras D, Pinset C. The glucocorticoid receptor and AP-1 are involved in a positive regulation of the muscle regulatory gene Myf5 in cultured myoblasts. J Cell Sci. 1997;110:2771–2779. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

