Relative importance of surface wettability and charged functional groups on NIH 3T3 fibroblast attachment, spreading, and cytoskeletal organization
- PMID: 9659612
- PMCID: PMC2632339
- DOI: 10.1002/(sici)1097-4636(19980905)41:3<422::aid-jbm12>3.0.co;2-k
Relative importance of surface wettability and charged functional groups on NIH 3T3 fibroblast attachment, spreading, and cytoskeletal organization
Abstract
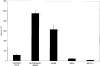
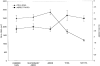
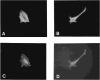
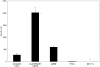
Understanding the relationships between material surface properties, adsorbed proteins, and cellular responses is essential to designing optimal material surfaces for implantation and tissue engineering. In this study, we have prepared model surfaces with different functional groups to provide a range of surface wettability and charge. The cellular responses of attachment, spreading, and cytoskeletal organization have been studied following preadsorption of these surfaces with dilute serum, specific serum proteins, and individual components of the extracellular matrix. When preadsorbed with dilute serum, cell attachment, spreading, and cytoskeletal organization were significantly greater on hydrophilic surfaces relative to hydrophobic surfaces. Among the hydrophilic surfaces, differences in charge and wettability influenced cell attachment but not cell area, shape, or cytoskeletal organization. Moderately hydrophilic surfaces (20-40 degree water contact angle) promoted the highest levels of cell attachment. Preadsorption of the model surfaces with bovine serum albumin (BSA) resulted in a pattern of cell attachment very similar to that observed following preadsorption with dilute serum, suggesting an important role for BSA in regulating cell attachment to biomaterials exposed to complex biological media.
Figures


Similar articles
-
Adhesion of mouse fibroblasts on hexamethyldisiloxane surfaces with wide range of wettability.J Biomed Mater Res B Appl Biomater. 2007 Apr;81(1):66-75. doi: 10.1002/jbm.b.30638. J Biomed Mater Res B Appl Biomater. 2007. PMID: 16924616
-
Relationships among cell attachment, spreading, cytoskeletal organization, and migration rate for anchorage-dependent cells on model surfaces.J Biomed Mater Res. 2000 Mar 5;49(3):362-8. doi: 10.1002/(sici)1097-4636(20000305)49:3<362::aid-jbm9>3.0.co;2-s. J Biomed Mater Res. 2000. PMID: 10602069 Free PMC article.
-
Adhesion of human peripheral blood lymphocytes is dependent on surface wettability and protein preadsorption.Biomaterials. 1994 May;15(6):423-8. doi: 10.1016/0142-9612(94)90220-8. Biomaterials. 1994. PMID: 8080932
-
Bioinspired surfaces with wettability for antifouling application.Nanoscale. 2019 Dec 21;11(47):22636-22663. doi: 10.1039/c9nr05870b. Epub 2019 Nov 22. Nanoscale. 2019. PMID: 31755511 Review.
-
Bioinspired surfaces with wettability: biomolecule adhesion behaviors.Biomater Sci. 2020 Mar 17;8(6):1502-1535. doi: 10.1039/c9bm01729a. Biomater Sci. 2020. PMID: 31994566 Review.
Cited by
-
Soy Protein Nanofiber Scaffolds for Uniform Maturation of Human Induced Pluripotent Stem Cell-Derived Retinal Pigment Epithelium.Tissue Eng Part C Methods. 2020 Aug;26(8):433-446. doi: 10.1089/ten.TEC.2020.0072. Tissue Eng Part C Methods. 2020. PMID: 32635833 Free PMC article.
-
Zn- or Cu-containing CaP-Based Coatings Formed by Micro-Arc Oxidation on Titanium and Ti-40Nb Alloy: Part II-Wettability and Biological Performance.Materials (Basel). 2020 Sep 30;13(19):4366. doi: 10.3390/ma13194366. Materials (Basel). 2020. PMID: 33008055 Free PMC article.
-
3D Fabrication and Characterisation of Electrically Receptive PCL-Graphene Scaffolds for Bioengineered In Vitro Tissue Models.Materials (Basel). 2022 Dec 17;15(24):9030. doi: 10.3390/ma15249030. Materials (Basel). 2022. PMID: 36556835 Free PMC article.
-
Comparison of physical, chemical and cellular responses to nano- and micro-sized calcium silicate/poly(epsilon-caprolactone) bioactive composites.J R Soc Interface. 2008 Jun 6;5(23):617-30. doi: 10.1098/rsif.2007.1267. J R Soc Interface. 2008. PMID: 17999948 Free PMC article.
-
Interaction of an ionic complementary peptide with a hydrophobic graphite surface.Protein Sci. 2010 Sep;19(9):1639-48. doi: 10.1002/pro.444. Protein Sci. 2010. PMID: 20572020 Free PMC article.
References
-
- Burridge K, Fath K, Kelly T, Nuckolls G, Turner C. Focal adhesions: Transmembrane junctions between the extracellular matrix and the cytoskeleton. Ann. Rev. Cell Biol. 1988;4:487–525. - PubMed
-
- Clark EA, Brugge JS. Integrins and signal transduction pathways: The road taken. Science. 1995;268:233–239. - PubMed
-
- Schwartz MA, Schaller MD, Ginsberg MH. Integrins: Emerging paradigms of signal transduction. Ann. Rev. Cell Dev. Biol. 1995;11:549–599. - PubMed
-
- Andrade JD, Hlady V. Protein adsorption and materials biocompatibility: A tutorial review and suggested hypotheses. Adv. Polym. Sci. 1986;79:1–63.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

