The LDL receptor clustering motif interacts with the clathrin terminal domain in a reverse turn conformation
- PMID: 9660863
- PMCID: PMC2133019
- DOI: 10.1083/jcb.142.1.59
The LDL receptor clustering motif interacts with the clathrin terminal domain in a reverse turn conformation
Abstract
Previously the hexapeptide motif FXNPXY807 in the cytoplasmic tail of the LDL receptor was shown to be essential for clustering in clathrin-coated pits. We used nuclear magnetic resonance line-broadening and transferred nuclear Overhauser effect measurements to identify the molecule in the clathrin lattice that interacts with this hexapeptide, and determined the structure of the bound motif. The wild-type peptide bound in a single conformation with a reverse turn at residues NPVY. Tyr807Ser, a peptide that harbors a mutation that disrupts receptor clustering, displayed markedly reduced interactions. Clustering motif peptides interacted with clathrin cages assembled in the presence or absence of AP2, with recombinant clathrin terminal domains, but not with clathrin hubs. The identification of terminal domains as the primary site of interaction for FXNPXY807 suggests that adaptor molecules are not required for receptor-mediated endocytosis of LDL, and that at least two different tyrosine-based internalization motifs exist for clustering receptors in coated pits.
Figures


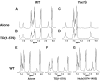
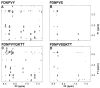
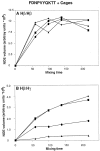



Similar articles
-
ARH is a modular adaptor protein that interacts with the LDL receptor, clathrin, and AP-2.J Biol Chem. 2002 Nov 15;277(46):44044-9. doi: 10.1074/jbc.M208539200. Epub 2002 Sep 8. J Biol Chem. 2002. PMID: 12221107
-
The modular adaptor protein autosomal recessive hypercholesterolemia (ARH) promotes low density lipoprotein receptor clustering into clathrin-coated pits.J Biol Chem. 2005 Dec 9;280(49):40996-1004. doi: 10.1074/jbc.M509394200. Epub 2005 Sep 22. J Biol Chem. 2005. PMID: 16179341
-
Disabled-2 colocalizes with the LDLR in clathrin-coated pits and interacts with AP-2.Traffic. 2001 Feb;2(2):111-23. doi: 10.1034/j.1600-0854.2001.020206.x. Traffic. 2001. PMID: 11247302
-
Clathrin coat construction in endocytosis.Curr Opin Struct Biol. 2000 Apr;10(2):220-8. doi: 10.1016/s0959-440x(00)00071-3. Curr Opin Struct Biol. 2000. PMID: 10753805 Review.
-
Clathrin and adaptors.Biochim Biophys Acta. 1998 Aug 14;1404(1-2):173-93. doi: 10.1016/s0167-4889(98)00056-1. Biochim Biophys Acta. 1998. PMID: 9714795 Review.
Cited by
-
Adaptor complex-independent clathrin function in yeast.Mol Biol Cell. 1999 Nov;10(11):3643-59. doi: 10.1091/mbc.10.11.3643. Mol Biol Cell. 1999. PMID: 10564262 Free PMC article.
-
Identification of a recurrent insertion mutation in the LDLR gene in a Pakistani family with autosomal dominant hypercholesterolemia.Mol Biol Rep. 2010 Dec;37(8):3869-75. doi: 10.1007/s11033-010-0043-0. Epub 2010 Mar 10. Mol Biol Rep. 2010. PMID: 20217239
-
Clathrin-mediated endocytosis in AP-2-depleted cells.J Cell Biol. 2003 Sep 1;162(5):909-18. doi: 10.1083/jcb.200305145. J Cell Biol. 2003. PMID: 12952941 Free PMC article.
-
Inhibition of endocytic pathways impacts cytomegalovirus maturation.Sci Rep. 2017 Apr 13;7:46069. doi: 10.1038/srep46069. Sci Rep. 2017. PMID: 28406138 Free PMC article.
-
Functional roles of short sequence motifs in the endocytosis of membrane receptors.Front Biosci (Landmark Ed). 2009 Jun 1;14(14):5339-60. doi: 10.2741/3599. Front Biosci (Landmark Ed). 2009. PMID: 19482617 Free PMC article. Review.
References
-
- Anderson RGW, Goldstein JL, Brown MS. A mutation that impairs the ability of lipoprotein receptors to localize in coated pits on the cell surface of human fibroblasts. Nature. 1976;270:695–699. - PubMed
-
- Bansal A, Gierasch LM. The NPXY internalization signal of the LDL receptor adopts a reverse-turn conformation. Cell. 1991;67:1195–1201. - PubMed
-
- Bax A, Grzesiek S. Methodological advances in protein NMR. Acc Chem Res. 1993;26:132–138.
-
- Bomsel M, de Paillerets C, Weintraub H, Alfsen A. Lipid bilayer dynamics in plasma and coated vesicle membranes from bovine adrenal cortex. Evidence of two types of coated vesicle involved in the LDL receptor traffic. Biochim Biophys Acta. 1986;859:15–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

