Functional specialization of stable and dynamic microtubules in protein traffic in WIF-B cells
- PMID: 9660870
- PMCID: PMC2133029
- DOI: 10.1083/jcb.142.1.153
Functional specialization of stable and dynamic microtubules in protein traffic in WIF-B cells
Abstract
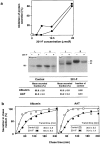
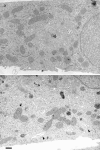
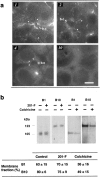
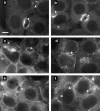
We found that the magnesium salt of ilimaquinone, named 201-F, specifically disassembled dynamically unstable microtubules in fibroblasts and various epithelial cell lines. Unlike classical tubulin- interacting drugs such as nocodazole or colchicine which affect all classes of microtubules, 201-F did not depolymerize stable microtubules. In WIF-B-polarized hepatic cells, 201-F disrupted the Golgi complex and inhibited albumin and alpha1-antitrypsin secretion to the same extent as nocodazole. By contrast, 201-F did not impair the transport of membrane proteins to the basolateral surface, which was only affected by the total disassembly of cellular microtubules. Transcytosis of two apical membrane proteins-the alkaline phosphodiesterase B10 and dipeptidyl peptidase IV-was affected to the same extent by 201-F and nocodazole. Taken together, these results indicate that only dynamically unstable microtubules are involved in the transport of secretory proteins to the plasma membrane, and in the transcytosis of membrane proteins to the apical surface. By contrast, stable microtubules, which are not functionally affected by 201-F treatment, are involved in the transport of membrane proteins to the basolateral surface. By specifically disassembling highly dynamic microtubules, 201-F is an invaluable tool with which to study the functional specialization of stable and dynamic microtubules in living cells.
Figures






References
-
- Biou D, Monnet D, Millet D, Feger J, Durand G. An immunochemical procedure to evaluate the degree of desialylation of alpha1-acid glycoprotein in rat serum. J Immunol Methods. 1984;74:267–271. - PubMed
-
- Carlson J, Stenflo J. The biosynthesis of rat alpha-1-antitrypsin. J Biol Chem. 1982;257:12987–12994. - PubMed

