STOP proteins are responsible for the high degree of microtubule stabilization observed in neuronal cells
- PMID: 9660871
- PMCID: PMC2133033
- DOI: 10.1083/jcb.142.1.167
STOP proteins are responsible for the high degree of microtubule stabilization observed in neuronal cells
Abstract
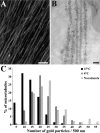
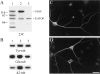
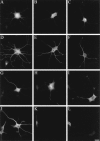
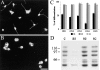
Neuronal differentiation and function require extensive stabilization of the microtubule cytoskeleton. Neurons contain a large proportion of microtubules that resist the cold and depolymerizing drugs and exhibit slow subunit turnover. The origin of this stabilization is unclear. Here we have examined the role of STOP, a calmodulin-regulated protein previously isolated from cold-stable brain microtubules. We find that neuronal cells express increasing levels of STOP and of STOP variants during differentiation. These STOP proteins are associated with a large proportion of microtubules in neuronal cells, and are concentrated on cold-stable, drug-resistant, and long-lived polymers. STOP inhibition abolishes microtubule cold and drug stability in established neurites and impairs neurite formation. Thus, STOP proteins are responsible for microtubule stabilization in neurons, and are apparently required for normal neurite formation.
Figures




References
-
- Baas PW, Pienkowski TP, Cimbalnik KA, Toyama K, Bakalis S, Ahmad FJ, Kosik KS. Tau confers drug stability but not cold stability to microtubules in living cells. J Cell Sci. 1994;107:135–143. - PubMed
-
- Barra HS, Arce CA, Argaraña CE. Posttranslational tyrosination/detyrosination of tubulin. Mol Neurobiol. 1988;2:133–153. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

