Targeting, capture, and stabilization of microtubules at early focal adhesions
- PMID: 9660872
- PMCID: PMC2133026
- DOI: 10.1083/jcb.142.1.181
Targeting, capture, and stabilization of microtubules at early focal adhesions
Abstract
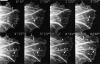
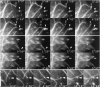
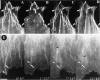
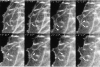
By co-injecting fluorescent tubulin and vinculin into fish fibroblasts we have revealed a "cross talk" between microtubules and early sites of substrate contact. This mutuality was first indicated by the targeting of vinculin-rich foci by microtubules during their growth towards the cell periphery. In addition to passing directly over contact sites, the ends of single microtubules could be observed to target several contacts in succession or the same contact repetitively, with intermittent withdrawals. Targeting sometimes involved side-stepping, or the major re-routing of a microtubule, indicative of a guided, rather than a random process. The paths that microtubules followed into contacts were unrelated to the orientation of stress fiber assemblies and targeting occurred also in mouse fibroblasts that lacked a system of intermediate filaments. Further experiments with microtubule inhibitors showed that adhesion foci can: (a) capture microtubules and stabilize them against disassembly by nocodazole; and (b), act as preferred sites of microtubule polymerization, during either early recovery from nocodazole, or brief treatment with taxol. From these and other findings we speculate that microtubules are guided into substrate contact sites and through the motor-dependent delivery of signaling molecules serve to modulate their development. It is further proposed this modulation provides the route whereby microtubules exert their influence on cell shape and polarity.
Figures






References
-
- Bershadsky A, Chausovsky A, Becker E, Lyubimova A, Geiger B. Involvement of microtubules in the control of adhesion-dependent signal transduction. Curr Biol. 1996;6:1279–1289. - PubMed
-
- Bershadsky AD, Vaisberg EA, Vasiliev JM. Pseudopodial activity at the active edge of migrating fibroblast is decreased after drug-induced microtubule depolymerization. Cell Motil Cytoskeleton. 1991;19:152–158. - PubMed
-
- Bershadsky, A.D., and J.M. Vasiliev. 1986. Cytoskeleton. Plenum Press, New York/London. 298 pp.
-
- Colucci-Guyon E, Portier MM, Dunia I, Paulin D, Pounin S, Babinet C. Mice lacking vimentin develop and reproduce without an obvious phenotype. Cell. 1994;79:679–694. - PubMed

