Regulation of angiotensin II-induced neuromodulation by MARCKS in brain neurons
- PMID: 9660875
- PMCID: PMC2133039
- DOI: 10.1083/jcb.142.1.217
Regulation of angiotensin II-induced neuromodulation by MARCKS in brain neurons
Abstract
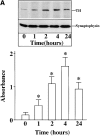
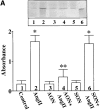
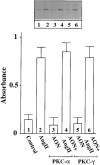
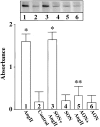
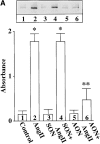
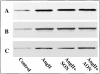
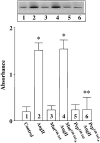
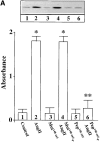
Angiotensin II (Ang II) exerts chronic stimulatory actions on tyrosine hydroxylase (TH), dopamine beta-hydroxylase (DbetaH), and the norepinephrine transporter (NET), in part, by influencing the transcription of their genes. These neuromodulatory actions of Ang II involve Ras-Raf-MAP kinase signal transduction pathways (Lu, D., H. Yang, and M.K. Raizada. 1997. J. Cell Biol. 135:1609-1617). In this study, we present evidence to demonstrate participation of another signaling pathway in these neuronal actions of Ang II. It involves activation of protein kinase C (PKC)beta subtype and phosphorylation and redistribution of myristoylated alanine-rich C kinase substrate (MARCKS) in neurites. Ang II caused a dramatic redistribution of MARCKS from neuronal varicosities to neurites. This was accompanied by a time-dependent stimulation of its phosphorylation, that was mediated by the angiotensin type 1 receptor subtype (AT1). Incubation of neurons with PKCbeta subtype specific antisense oligonucleotide (AON) significantly attenuated both redistribution and phosphorylation of MARCKS. Furthermore, depletion of MARCKS by MARCKS-AON treatment of neurons resulted in a significant decrease in Ang II-stimulated accumulation of TH and DbetaH immunoreactivities and [3H]NE uptake activity in synaptosomes. In contrast, mRNA levels of TH, DbetaH, and NET were not influenced by MARKS-AON treatment. MARCKS pep148-165, which contains PKC phosphorylation sites, inhibited Ang II stimulation of MARCKS phosphorylation and reduced the amount of TH, DbetaH, and [3H]NE uptake in neuronal synaptosomes. These observations demonstrate that phosphorylation of MARCKS by PKCbeta and its redistribution from varicosities to neurites is important in Ang II-induced synaptic accumulation of TH, DbetaH, and NE. They suggest that a coordinated stimulation of transcription of TH, DbetaH, and NET, mediated by Ras-Raf-MAP kinase followed by their transport mediated by PKCbeta-MARCKS pathway are key in persistent stimulation of Ang II's neuromodulatory actions.
Figures

















References
-
- Aderem A. The MARCKS family of protein kinase-C substrates. Biochem Soc Trans. 1995;23:587–591. - PubMed
-
- Ahmad F, Li PM, Meyerovitch J, Goldstein BJ. Osmotic loading of neutralizing antibodies demonstrates a role for protein-tyrosine phosphatase 1B in negative regulation of the insulin action pathway. J Biol Chem. 1995;270:20503–20508. - PubMed
-
- Amess B, Manjarrez-Hernandex HA, Howell SA, Learmonth M, Aitken A. Multisite phosphorylation of the 80 kDa (MARCKS) protein kinase C substrate in C3H/10T1/Z fibroblasts, quantitative analysis of individual sites by solid-phase microsequencing. FEBS (Fed Eur Biochem Soc) Lett. 1992;297:285–291. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

