Dimerization of P-selectin glycoprotein ligand-1 (PSGL-1) required for optimal recognition of P-selectin
- PMID: 9660879
- PMCID: PMC2133017
- DOI: 10.1083/jcb.142.1.263
Dimerization of P-selectin glycoprotein ligand-1 (PSGL-1) required for optimal recognition of P-selectin
Abstract
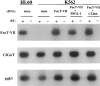
Interactions between P-selectin, expressed on endothelial cells and activated platelets, and its leukocyte ligand, a homodimer termed P-selectin glycoprotein ligand-1 (PSGL-1), mediate the earliest adhesive events during an inflammatory response. To investigate whether dimerization of PSGL-1 is essential for functional interactions with P-selectin, a mutant form of PSGL-1 was generated in which the conserved membrane proximal cysteine was mutated to alanine (designated C320A). Western blotting under both denaturing and native conditions of the C320A PSGL-1 mutant isolated from stably transfected cells revealed expression of only a monomeric form of PSGL-1. In contrast to cells cotransfected with alpha1-3 fucosyltransferase-VII (FucT-VII) plus PSGL-1, K562 cells expressing FucT-VII plus C320A failed to bind COS cells transfected with P-selectin in a low shear adhesion assay, or to roll on CHO cells transfected with P-selectin under conditions of physiologic flow. In addition, C320A transfectants failed to bind chimeric P-selectin fusion proteins. Both PSGL-1 and C320A were uniformly distributed on the surface of transfected K562 cells. Thus, dimerization of PSGL-1 through the single, conserved, extracellular cysteine is essential for functional recognition of P-selectin.
Figures








Similar articles
-
Functional analysis of the combined role of the O-linked branching enzyme core 2 beta1-6-N-glucosaminyltransferase and dimerization of P-selectin glycoprotein ligand-1 in rolling on P-selectin.J Biol Chem. 2004 May 21;279(21):21984-91. doi: 10.1074/jbc.M402731200. Epub 2004 Mar 16. J Biol Chem. 2004. PMID: 15026421
-
Noncovalent association of P-selectin glycoprotein ligand-1 and minimal determinants for binding to P-selectin.J Biol Chem. 2000 Mar 17;275(11):7839-53. doi: 10.1074/jbc.275.11.7839. J Biol Chem. 2000. PMID: 10713099
-
P-selectin glycoprotein ligand-1 is essential for adhesion to P-selectin but not E-selectin in stably transfected hematopoietic cell lines.Blood. 1997 Feb 1;89(3):896-901. Blood. 1997. PMID: 9028320
-
Regulation of PSGL-1 interactions with L-selectin, P-selectin, and E-selectin: role of human fucosyltransferase-IV and -VII.J Biol Chem. 2005 Feb 18;280(7):5378-90. doi: 10.1074/jbc.M410899200. Epub 2004 Dec 3. J Biol Chem. 2005. PMID: 15579466
-
Functional characterization of L-selectin ligands on human neutrophils and leukemia cell lines: evidence for mucinlike ligand activity distinct from P-selectin glycoprotein ligand-1.Blood. 1998 Feb 1;91(3):1067-75. Blood. 1998. PMID: 9446670
Cited by
-
Two by two: the pairings of P-selectin and P-selectin glycoprotein ligand 1.Proc Natl Acad Sci U S A. 2001 Aug 28;98(18):10023-4. doi: 10.1073/pnas.191367898. Proc Natl Acad Sci U S A. 2001. PMID: 11526223 Free PMC article. No abstract available.
-
The PEN5 epitope identifies an oligodendrocyte precursor cell population and pilocytic astrocytomas.Am J Pathol. 1999 Oct;155(4):1261-9. doi: 10.1016/S0002-9440(10)65228-5. Am J Pathol. 1999. PMID: 10514408 Free PMC article.
-
The transmembrane domains of L-selectin and CD44 regulate receptor cell surface positioning and leukocyte adhesion under flow.J Biol Chem. 2010 Apr 30;285(18):13490-7. doi: 10.1074/jbc.M110.102640. Epub 2010 Mar 8. J Biol Chem. 2010. PMID: 20212041 Free PMC article.
-
Functional adhesiveness of the CX3CL1 chemokine requires its aggregation. Role of the transmembrane domain.J Biol Chem. 2008 Oct 31;283(44):30225-34. doi: 10.1074/jbc.M802638200. Epub 2008 Aug 25. J Biol Chem. 2008. PMID: 18725411 Free PMC article.
-
Molecular cloning and expression analysis of P-selectin glycoprotein ligand-1 from zebrafish (Danio rerio).Fish Physiol Biochem. 2012 Apr;38(2):555-64. doi: 10.1007/s10695-011-9535-7. Epub 2011 Jul 14. Fish Physiol Biochem. 2012. PMID: 21755364
References
-
- Breuhl RE, Moore KL, Lorant DE, Borregard N, Zimmerman GA, McEver RP, Bainton DF. Leukocyte activation induces surface redistribution of P-selectin glycoprotein ligand-1. J Leuk Biol. 1997;61:489–499. - PubMed
-
- Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. - PubMed
-
- Erlandsen SL, Hasslen SR, Nelson RD. Detection and spatial distribution of the β2 integrin (Mac-1) and L-selectin (LECAM-1) adherence receptors on human neutrophils by high resolution field emission SEM. J Histochem Cytochem. 1993;41:327–333. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

