Cartilage fibrils of mammals are biochemically heterogeneous: differential distribution of decorin and collagen IX
- PMID: 9660881
- PMCID: PMC2133020
- DOI: 10.1083/jcb.142.1.285
Cartilage fibrils of mammals are biochemically heterogeneous: differential distribution of decorin and collagen IX
Abstract
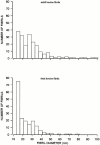
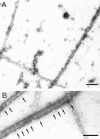
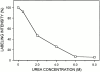
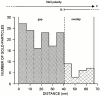
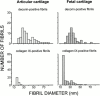
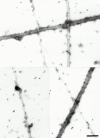
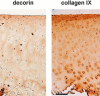
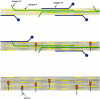
Cartilage fibrils contain collagen II as the major constituent, but the presence of additional components, minor collagens, and noncollagenous glycoproteins is thought to be crucial for modulating several fibril properties. We have examined the distribution of two fibril constituents-decorin and collagen IX-in samples of fibril fragments obtained after bovine cartilage homogenization. Decorin was preferentially associated with a population of thicker fibril fragments from adult articular cartilage, but was not present on the thinnest fibrils. The binding was specific for the gap regions of the fibrils, and depended on the decorin core protein. Collagen IX, by contrast, predominated in the population with the thinnest fibrils, and was scarce on wider fibrils. Double-labeling experiments demonstrated the coexistence of decorin and collagen IX in some fibrils of intermediate diameter, although most fibril fragments from adult cartilage were strongly positive for one component and lacked the other. Fibril fragments from fetal epiphyseal cartilage showed a different pattern, with decorin and collagen IX frequently colocalized on fragments of intermediate and large diameters. Hence, the presence of collagen IX was not exclusive for fibrils of small diameter. These results establish that articular cartilage fibrils are biochemically heterogeneous. Different populations of fibrils share collagen II, but have distinct compositions with respect to macromolecules defining their surface properties.
Figures




Similar articles
-
Light and electron microscopical immunohistochemical localization of the small proteoglycan core proteins decorin and biglycan in human knee joint cartilage.Histochem J. 1994 Dec;26(12):939-45. Histochem J. 1994. PMID: 7896570
-
Distinctive localization and function for lumican, fibromodulin and decorin to regulate collagen fibril organization in periodontal tissues.J Periodontal Res. 2005 Aug;40(4):312-24. doi: 10.1111/j.1600-0765.2005.00800.x. J Periodontal Res. 2005. PMID: 15966909
-
Axial structure of the heterotypic collagen fibrils of vitreous humour and cartilage.J Mol Biol. 2001 Mar 9;306(5):1011-22. doi: 10.1006/jmbi.2000.4429. J Mol Biol. 2001. PMID: 11237615
-
The collagens of articular cartilage.Semin Arthritis Rheum. 1991 Dec;21(3 Suppl 2):2-11. doi: 10.1016/0049-0172(91)90035-x. Semin Arthritis Rheum. 1991. PMID: 1796302 Review.
-
Collagen IX.Int J Biochem Cell Biol. 1997 Apr;29(4):555-8. doi: 10.1016/s1357-2725(96)00100-8. Int J Biochem Cell Biol. 1997. PMID: 9363632 Review.
Cited by
-
Recombinant Extracellular Matrix Protein Fragments Support Human Embryonic Stem Cell Chondrogenesis.Tissue Eng Part A. 2018 Jun;24(11-12):968-978. doi: 10.1089/ten.TEA.2017.0285. Epub 2018 Feb 7. Tissue Eng Part A. 2018. PMID: 29279011 Free PMC article.
-
Glycosaminoglycans in the pericellular matrix of chondrons and chondrocytes.J Anat. 2008 Sep;213(3):266-73. doi: 10.1111/j.1469-7580.2008.00942.x. Epub 2008 Jul 8. J Anat. 2008. PMID: 18631286 Free PMC article.
-
Decorin expression is important for age-related changes in tendon structure and mechanical properties.Matrix Biol. 2013 Jan;32(1):3-13. doi: 10.1016/j.matbio.2012.11.005. Epub 2012 Nov 23. Matrix Biol. 2013. PMID: 23178232 Free PMC article.
-
Sequential Zonal Chondrogenic Differentiation of Mesenchymal Stem Cells in Cartilage Matrices.Tissue Eng Part A. 2019 Feb;25(3-4):234-247. doi: 10.1089/ten.TEA.2018.0083. Epub 2018 Dec 28. Tissue Eng Part A. 2019. PMID: 30146939 Free PMC article.
-
Reduced cell proliferation and increased apoptosis are significant pathological mechanisms in a murine model of mild pseudoachondroplasia resulting from a mutation in the C-terminal domain of COMP.Hum Mol Genet. 2007 Sep 1;16(17):2072-88. doi: 10.1093/hmg/ddm155. Epub 2007 Jun 22. Hum Mol Genet. 2007. PMID: 17588960 Free PMC article.
References
-
- Birk DE, Nurminskaya MV, Zycband EI. Collagen fibrillogenesis in situ: fibril segments undergo post-depositional modifications resulting in linear and lateral growth during matrix development. Dev Dyn. 1995;202:229–243. - PubMed
-
- Brown DC, Vogel KG. Characteristics of the in vitro interaction of a small proteoglycan (PGII) of bovine tendon with type I collagen. Matrix. 1989;9:468–478. - PubMed
-
- Bruckner P, Mendler M, Steinmann B, Huber S, Winterhalter KH. The structure of human collagen type IX and its organization in fetal and infant cartilage fibrils. J Biol Chem. 1988;263:16911–16917. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

