Cartilage fibrils of mammals are biochemically heterogeneous: differential distribution of decorin and collagen IX
- PMID: 9660881
- PMCID: PMC2133020
- DOI: 10.1083/jcb.142.1.285
Cartilage fibrils of mammals are biochemically heterogeneous: differential distribution of decorin and collagen IX
Abstract
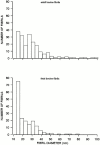
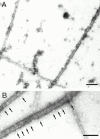
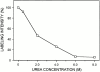
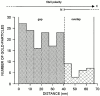
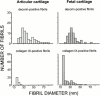
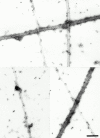
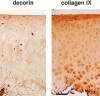
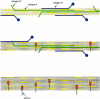
Cartilage fibrils contain collagen II as the major constituent, but the presence of additional components, minor collagens, and noncollagenous glycoproteins is thought to be crucial for modulating several fibril properties. We have examined the distribution of two fibril constituents-decorin and collagen IX-in samples of fibril fragments obtained after bovine cartilage homogenization. Decorin was preferentially associated with a population of thicker fibril fragments from adult articular cartilage, but was not present on the thinnest fibrils. The binding was specific for the gap regions of the fibrils, and depended on the decorin core protein. Collagen IX, by contrast, predominated in the population with the thinnest fibrils, and was scarce on wider fibrils. Double-labeling experiments demonstrated the coexistence of decorin and collagen IX in some fibrils of intermediate diameter, although most fibril fragments from adult cartilage were strongly positive for one component and lacked the other. Fibril fragments from fetal epiphyseal cartilage showed a different pattern, with decorin and collagen IX frequently colocalized on fragments of intermediate and large diameters. Hence, the presence of collagen IX was not exclusive for fibrils of small diameter. These results establish that articular cartilage fibrils are biochemically heterogeneous. Different populations of fibrils share collagen II, but have distinct compositions with respect to macromolecules defining their surface properties.
Figures




References
-
- Birk DE, Nurminskaya MV, Zycband EI. Collagen fibrillogenesis in situ: fibril segments undergo post-depositional modifications resulting in linear and lateral growth during matrix development. Dev Dyn. 1995;202:229–243. - PubMed
-
- Brown DC, Vogel KG. Characteristics of the in vitro interaction of a small proteoglycan (PGII) of bovine tendon with type I collagen. Matrix. 1989;9:468–478. - PubMed
-
- Bruckner P, Mendler M, Steinmann B, Huber S, Winterhalter KH. The structure of human collagen type IX and its organization in fetal and infant cartilage fibrils. J Biol Chem. 1988;263:16911–16917. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

