Angiotensin AT1 receptor-mediated excitation of rat carotid body chemoreceptor afferent activity
- PMID: 9660892
- PMCID: PMC2231066
- DOI: 10.1111/j.1469-7793.1998.773bj.x
Angiotensin AT1 receptor-mediated excitation of rat carotid body chemoreceptor afferent activity
Abstract
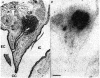
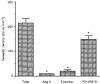
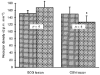
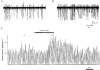
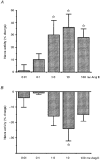
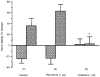
1. A high density of angiotensin II receptors was observed in the rat carotid body by in vitro autoradiography employing 125I-[Sar1, Ile8]-angiotensin II as radioligand. Displacement studies demonstrated that the receptors were of the AT1 subtype. 2. The binding pattern indicated that the AT1 receptors occurred over clumps of glomus cells, the principal chemoreceptor cell of the carotid body. Selective lesions of the sympathetic or afferent innervation of the carotid body had little effect on the density of receptor binding, demonstrating that the majority of AT1 receptors were intrinsic to the glomus cells. 3. To determine the direct effect of angiotensin II on chemoreceptor function, without the confounding effects of the vasoconstrictor action of angiotensin II, carotid sinus nerve activity was recorded from the isolated carotid body in vitro. The carotid body was superfused with Tyrode solution saturated with carbogen (95 % O2, 5 % CO2), maintained at 36 C, and multi-unit nerve activity recorded with a suction electrode. 4. Angiotensin II elicited a dose-dependent excitation of carotid sinus nerve activity (maximum increase of 36 +/- 11 % with 10 nM angiotensin II) with a threshold concentration of 1 nM. The response was blocked by the addition of an AT1 receptor antagonist, losartan (1 microM), but not by the addition of an AT2 receptor antagonist, PD123319 (1 microM). 5. In approximately 50 % of experiments the excitation was preceded by an inhibition of activity (maximum decrease of 24 +/- 8 % with 10 nM angiotensin II). This inhibitory response was markedly attenuated by losartan but not affected by PD123319. 6. These observations demonstrate that angiotensin II, acting through AT1 receptors located on glomus cells in the carotid body, can directly alter carotid chemoreceptor afferent activity. This provides a means whereby humoral information about fluid and electrolyte homeostasis might influence control of cardiorespiratory function.
Figures
References
-
- Allen AM, Chai SY, Clevers J, McKinley MJ, Paxinos G, Mendelsohn FAO. Localization and characterization of angiotensin II receptor binding and angiotensin converting enzyme in the human medulla oblongata. Journal of Comparative Neurology. 1988;269:249–264. - PubMed
-
- Allen AM, Paxinos G, McKinley MJ, Chai SY, Mendelsohn FAO. Localization and characterization of angiotensin II receptor binding sites in the human basal ganglia, thalamus, midbrain, pons and cerebellum. Journal of Comparative Neurology. 1991;312:291–298. - PubMed
-
- Assmussen E, Nielsen M. Studies on the initial increase in O2-capacity of the blood at low oxygen pressure. Acta Physiologica Scandinavica. 1945;9:75–87.
-
- Castren E, Kurihara M, Gutkind JS, Saavedra JM. Specific angiotensin binding sites in the rat stellate and superior cervical ganglia. Brain Research. 1987;422:347–351. - PubMed
-
- Donnelly DF. Does catecholamine secretion mediate the hypoxia-induced increase in nerve activity? Biological Signals. 1995;4:304–309. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

