Characterization of sensory and corticothalamic excitatory inputs to rat thalamocortical neurones in vitro
- PMID: 9660897
- PMCID: PMC2231073
- DOI: 10.1111/j.1469-7793.1998.829bj.x
Characterization of sensory and corticothalamic excitatory inputs to rat thalamocortical neurones in vitro
Abstract
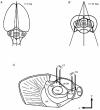
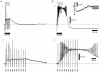
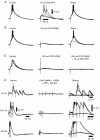
1. Using an in vitro slice preparation of the rat dorsal lateral geniculate nucleus (dLGN), the properties of retinogeniculate and corticothalamic inputs to thalamocortical (TC) neurones were examined in the absence of GABAergic inhibition. 2. The retinogeniculate EPSP evoked at low frequency (>= 0.1 Hz) consisted of one or two fast-rising (0.8 +/- 0.1 ms), large-amplitude (10.3 +/- 1.6 mV) unitary events, while the corticothalamic EPSP had a graded relationship with stimulus intensity, owing to its slower-rising (2.9 +/- 0.4 ms), smaller-amplitude (1.3 +/- 0.3 mV) estimated unitary components. 3. The retinogeniculate EPSP exhibited a paired-pulse depression of 60.3 +/- 5.6 % at 10 Hz, while the corticothalamic EPSP exhibited a paired-pulse facilitation of > 150 %. This frequency-dependent depression of the retinogeniculate EPSP was maximal after the second stimulus, while the frequency-dependent facilitation of the corticothalamic EPSP was maximal after the fourth or fifth stimulus, at interstimulus frequencies of 1-10 Hz. 4. There was a short-term enhancement of the >= 0.1 Hz corticothalamic EPSP (64.6 +/- 9.2 %), but not the retinogeniculate EPSP, following trains of stimuli at 50 Hz. 5. The >= 0.1 Hz corticothalamic EPSP was markedly depressed by the non-NMDA antagonist 1-(4-amino-phenyl)-4-methyl-7,8-methylene-dioxy-5H-2, 3-benzodiazepine (GYKI 52466), but only modestly by the NMDA antagonist 3-((RS)-2-carboxypiperazin-4-yl)-propyl-1-phosphonic acid ((RS)-CPP), and completely blocked by the co-application of GYKI 52466, 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), (RS)-CPP and (5R, 10S)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5, 10-imine (MK-801). Likewise, the corticothalamic responses to trains of stimuli (1-500 Hz) were greatly reduced by this combination of ionotropic glutamate receptor antagonists. 6. In the presence of GYKI 52466, CNQX, (RS)-CPP and MK-801, residual corticothalamic responses and slow EPSPs, with a time to peak of 2-10 s, could be generated following trains of five to fifty stimuli. Neither of these responses were occluded by 1S,3R-1-aminocyclopentane-1, 3-dicarboxylic acid (1S,3R-ACPD), suggesting they are not mediated via group I and II metabotropic glutamate receptors.
Figures





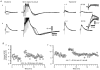

Similar articles
-
Physiology and pharmacology of corticothalamic stimulation-evoked responses in rat somatosensory thalamic neurons in vitro.J Neurophysiol. 1997 May;77(5):2661-76. doi: 10.1152/jn.1997.77.5.2661. J Neurophysiol. 1997. PMID: 9163382
-
Sensory input and burst firing output of rat and cat thalamocortical cells: the role of NMDA and non-NMDA receptors.J Physiol. 1994 Oct 15;480 ( Pt 2)(Pt 2):281-95. doi: 10.1113/jphysiol.1994.sp020359. J Physiol. 1994. PMID: 7869244 Free PMC article.
-
Dynamic properties of corticothalamic excitatory postsynaptic potentials and thalamic reticular inhibitory postsynaptic potentials in thalamocortical neurons of the guinea-pig dorsal lateral geniculate nucleus.Neuroscience. 1999;91(1):7-20. doi: 10.1016/s0306-4522(98)00557-0. Neuroscience. 1999. PMID: 10336055
-
The multifaceted role of inhibitory interneurons in the dorsal lateral geniculate nucleus.Vis Neurosci. 2017 Jan;34:E017. doi: 10.1017/S0952523817000141. Vis Neurosci. 2017. PMID: 28965520 Review.
-
The action of the corticofugal pathway on sensory thalamic nuclei: a hypothesis.Neuroscience. 1987 Nov;23(2):399-406. doi: 10.1016/0306-4522(87)90064-9. Neuroscience. 1987. PMID: 2830559 Review.
Cited by
-
A corticothalamic switch: controlling the thalamus with dynamic synapses.Neuron. 2015 May 6;86(3):768-82. doi: 10.1016/j.neuron.2015.03.040. Epub 2015 Apr 23. Neuron. 2015. PMID: 25913856 Free PMC article.
-
Passive Synaptic Normalization and Input Synchrony-Dependent Amplification of Cortical Feedback in Thalamocortical Neuron Dendrites.J Neurosci. 2016 Mar 30;36(13):3735-54. doi: 10.1523/JNEUROSCI.3836-15.2016. J Neurosci. 2016. PMID: 27030759 Free PMC article.
-
Energy-Efficient Information Transfer by Visual Pathway Synapses.Curr Biol. 2015 Dec 21;25(24):3151-60. doi: 10.1016/j.cub.2015.10.063. Epub 2015 Dec 6. Curr Biol. 2015. PMID: 26671670 Free PMC article.
-
A novel mechanism for short-term post-tetanic plasticity in thalamocortical neurons.Brain Res. 2025 Jul 15;1859:149654. doi: 10.1016/j.brainres.2025.149654. Epub 2025 Apr 21. Brain Res. 2025. PMID: 40268039
-
Reciprocal interaction between cortical SST and PV interneurons in top-down regulation of retinothalamic refinement.Proc Natl Acad Sci U S A. 2025 Jun 24;122(25):e2504224122. doi: 10.1073/pnas.2504224122. Epub 2025 Jun 18. Proc Natl Acad Sci U S A. 2025. PMID: 40531875
References
-
- Aghajanian GK, Rasmussen K. Intracellular studies in the facial nucleus illustrating a simple new method for obtaining viable motoneurons in adult rat brain slices. Synapse. 1989;3:331–338. - PubMed
-
- Bloomfield SA, Sherman SM. Postsynaptic potentials recorded in neurons of the cat's lateral geniculate nucleus following electrical stimulation of the optic chiasm. Journal of Neurophysiology. 1988;60:1924–1945. - PubMed
-
- Bourassa J, Deschênes M. Corticothalamic projections from the primary visual cortex in rats: a single fiber study using biocytin as an antegrade tracer. Neuroscience. 1995;66:253–263. - PubMed
-
- Castro-Alamancos MA, Landisman CE, Connors BW. Mechanisms of facilitation at corticothalamic synapses in the ventrobasal thalamus. Society for Neuroscience Abstracts. 1997;23:230.5.
-
- Contreras D, Destexhe A, Sejnowski TJ, Steriade M. Control of spatiotemporal coherence of a thalamic oscillation by corticothalamic feedback. Science. 1996;274:771–774. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

