Mechanistic analyses of ion dependences in a high-affinity human serotonin transport system in transfected murine fibroblast cells
- PMID: 9660901
- PMCID: PMC2231071
- DOI: 10.1111/j.1469-7793.1998.903bj.x
Mechanistic analyses of ion dependences in a high-affinity human serotonin transport system in transfected murine fibroblast cells
Abstract
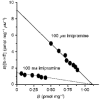
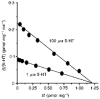
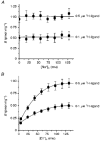
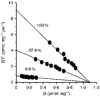
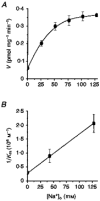
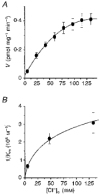
1. A clonal cell line, L-S1, has been identified from transfection of human genomic DNA into cultured mouse L-M fibroblasts. Because this transfectant cell line stably expresses a high-affinity serotonin (5-HT) transport mechanism with kinetic and pharmacological properties comparable to those of other serotonin uptake systems, it was used to investigate the mechanistic involvement of Na+ and Cl- ions in the ligand binding and kinetic uptake processes of this system. 2. Intact transfectant cells, when incubated at low temperature (4 C), enabled quantitative assessment of imipramine-displaceable 5-[3H]HT binding to the 5-HT transport system. This binding activity is insensitive to the presence of various ligands specific for 5-HT receptor subtypes. 3. Imipramine-displaceable 5-[3H]HT binding to intact L-S1 cells was shown to be a Cl--dependent but Na+-independent process. Chloride ions lack binding co-operativity in facilitating ligand binding. Changes in external Cl- concentration altered the Kd but not the Bmax of binding. 4. The overall transport activity was observed to be highly dependent on both external Na+ and Cl- concentrations, characterized by a 5-HT:Na+:Cl- coupling ratio of 1:1:1 per transport cycle. Alterations in the external concentrations of both Na+ and Cl- ions altered only the Km and not the Vmax of transport. 5. Both binding and kinetic results are consistent with kinetic modelling predictions of the Cl- ion in facilitating 5-HT binding to the transport system, and of the Na+ ion in enabling translocation of bound 5-HT across the plasma membrane. Thus, Na+ and Cl- ions facilitate mechanistically distinct and discernible functions in the transport cycle.
Figures
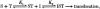
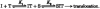
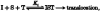
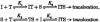
Similar articles
-
Ligand binding to the serotonin transporter: equilibria, kinetics, and ion dependence.Biochemistry. 1994 Aug 9;33(31):9118-25. doi: 10.1021/bi00197a014. Biochemistry. 1994. PMID: 8049215
-
Characterization of a genetically reconstituted high-affinity system for serotonin transport.Proc Natl Acad Sci U S A. 1989 Dec;86(23):9611-5. doi: 10.1073/pnas.86.23.9611. Proc Natl Acad Sci U S A. 1989. PMID: 2594789 Free PMC article.
-
Affinities of venlafaxine and various reuptake inhibitors for the serotonin and norepinephrine transporters.Eur J Pharmacol. 1998 May 15;349(1):129-32. doi: 10.1016/s0014-2999(98)00241-6. Eur J Pharmacol. 1998. PMID: 9669506
-
Substrate and inhibitor binding and translocation by the platelet plasma membrane serotonin transporter.Biochem Soc Trans. 1991 Feb;19(1):95-8. doi: 10.1042/bst0190095. Biochem Soc Trans. 1991. PMID: 2037207 Review.
-
Impact of oligomerization on the function of the human serotonin transporter.Biochem Soc Trans. 2001 Nov;29(Pt 6):732-6. doi: 10.1042/0300-5127:0290732. Biochem Soc Trans. 2001. PMID: 11709065 Review.
Cited by
-
A conserved asparagine residue in transmembrane segment 1 (TM1) of serotonin transporter dictates chloride-coupled neurotransmitter transport.J Biol Chem. 2011 Sep 2;286(35):30823-30836. doi: 10.1074/jbc.M111.250308. Epub 2011 Jul 5. J Biol Chem. 2011. PMID: 21730057 Free PMC article.
-
The two Na+ sites in the human serotonin transporter play distinct roles in the ion coupling and electrogenicity of transport.J Biol Chem. 2014 Jan 17;289(3):1825-40. doi: 10.1074/jbc.M113.504654. Epub 2013 Nov 29. J Biol Chem. 2014. PMID: 24293367 Free PMC article.
-
Catechol-O-methyltransferase activity in CHO cells expressing norepinephrine transporter.Br J Pharmacol. 1999 Oct;128(3):774-80. doi: 10.1038/sj.bjp.0702831. Br J Pharmacol. 1999. PMID: 10516661 Free PMC article.
-
GABA-induced current and circadian regulation of chloride in neurones of the rat suprachiasmatic nucleus.J Physiol. 2001 Dec 15;537(Pt 3):853-69. doi: 10.1111/j.1469-7793.2001.00853.x. J Physiol. 2001. PMID: 11744760 Free PMC article.
-
Uptake and metabolism of serotonin by ependymal primary cultures.Neurochem Res. 2004 Sep;29(9):1739-47. doi: 10.1023/b:nere.0000035810.08543.97. Neurochem Res. 2004. PMID: 15453270
References
-
- Amara SG, Kuhar MJ. Neurotransmitter transporters: Recent progress. Annual Review of Neuroscience. 1993;16:73–93. 10.1146/annurev.ne.16.030193.000445. - DOI - PubMed
-
- Amejdki-Chab N, Costentin J, Bonnet J-J. Kinetic analysis of the chloride dependence of the neuronal uptake of dopamine and effect of anions on the ability of substrates to compete with the binding of the dopamine uptake inhibitor GBR 12783. Journal of Neurochemistry. 1992;58:793–800. - PubMed
-
- Briley M, Langer SZ. Sodium-dependency of [3H]imipramine binding to rat cerebral cortex. European Journal of Pharmacology. 1981;72:377–380. - PubMed
-
- Chang AS, Chang SM, Starnes DM. Structure-activity relationships of serotonin transport: Relevance to nontricyclic antidepressant interactions. European Journal of Pharmacology. 1993;247:239–248. - PubMed
-
- Chang AS, Chang SM, Starnes DM, Schroeter S, Bauman AL, Blakely RD. Cloning and expression of the mouse serotonin transporter. Molecular Brain Research. 1996;43:185–192. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

