A cross-bridge mechanism can explain the thixotropic short-range elastic component of relaxed frog skeletal muscle
- PMID: 9660904
- PMCID: PMC2231083
- DOI: 10.1111/j.1469-7793.1998.941bj.x
A cross-bridge mechanism can explain the thixotropic short-range elastic component of relaxed frog skeletal muscle
Abstract
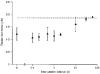
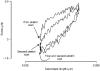
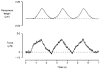
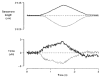
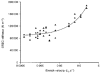
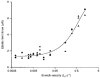
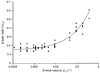
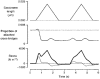
1. The passive tension and sarcomere length of relaxed frog skeletal muscle fibres were measured in response to imposed length stretches. The tension response to a constant-velocity stretch exhibited a clear discontinuity. Tension rose more rapidly during the initial approximately 0.4 % L0 of the stretch than during the latter stages (where L0 is the resting length of the fibre). This initial tension response is attributed to the short-range elastic component (SREC). 2. The use of paired triangular stretches revealed that the maximum tension produced during the SREC response of the second stretch was significantly reduced by the first stretch. This history-dependent behaviour of the SREC reflects thixotropic stiffness changes that have been previously described in relaxed muscle. 3. The biphasic nature of the SREC tension response to movement was most apparent during the first imposed length change after a period at a fixed length, irrespective of the direction of movement. 4. If a relaxed muscle was subjected to an imposed triangular length change so that the muscle was initially stretched and subsequently shortened back to its original fibre length, the resting tension at the end of the stretch was reduced relative to its initial pre-stretch value. Following the end of the stretch, tension slowly increased towards its initial value but the tension recovery was not accompanied by a contemporaneous increase in sarcomere length. This finding suggests that the resting tension of a relaxed muscle fibre is not entirely due to passive elasticity. The results are compatible with the suggestion that a portion of the resting tension - the filamentary resting tension (FRT) - is produced by a low level of active force generation. 5. If a second identical stretch was imposed on the muscle at a time when the resting tension was reduced by the previous stretch, the maximal tension produced during the second stretch was the same as that produced during the first, despite the second stretch commencing from a lower initial resting tension. 6. In experiments using paired triangular length changes, an inter-stretch interval of zero did not produce a substantially greater thixotropic reduction in the second stretch elastic limit force than an inter-stretch interval in the range 0.5-1 s. 7. A theoretical model was developed in which the SREC and FRT arise as manifestations of a small number of slowly cycling cross-bridges linking the actin and myosin filaments of a relaxed skeletal muscle. The predictions of the model are compatible with many of the experimental observations. If the SREC and FRT are indeed due to cross-bridge activity, the model suggests that the cross-bridge attachment rate must increase during interfilamentary movement. A mechanism (based on misregistration between the actin binding sites and the myosin cross-bridges) by which this might arise is presented.
Figures






References
-
- Bartoo ML, Linke WA, Pollack GH. Basis of passive tension and stiffness in isolated rabbit myofibrils. American Journal of Physiology. 1997;273:C266–276. - PubMed
-
- Brenner B. Muscle mechanics and biochemical kinetics. In: Squire JM, editor. Molecular Mechanisms in Muscular Contraction. London: Macmillan Press; 1990. pp. 77–150.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

