Antiretroviral efficacy and pharmacokinetics of oral bis(isopropyloxycarbonyloxymethyl)-9-(2-phosphonylmethoxypropyl)adenine in mice
- PMID: 9660984
- PMCID: PMC105646
- DOI: 10.1128/AAC.42.7.1568
Antiretroviral efficacy and pharmacokinetics of oral bis(isopropyloxycarbonyloxymethyl)-9-(2-phosphonylmethoxypropyl)adenine in mice
Abstract
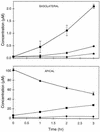
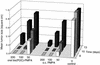
To overcome the low oral bioavailability of the highly potent and selective antiretroviral agent (R)-9-(2-phosphonylmethoxypropyl)adenine (PMPA), a new lipophilic ester derivative, i.e., the bis(isopropyloxycarbonyloxymethyl)-ester [bis(POC)-PMPA], was prepared. The usefulness of bis(POC)-PMPA as an oral prodrug for PMPA was investigated in the intestinal mucosa Caco-2 cell monolayer model. The total transport of bis(POC)-PMPA was 2.7%, whereas it was less than 0.1% for PMPA. Bis(POC)-PMPA was considerably metabolized inside the epithelial cells, since the majority of the compound was recovered after transport in the form of the monoester metabolite [mono(POC)-PMPA]. In contrast, bis(POC)-PMPA was relatively resistant to degradation at the luminal side of the Caco-2 cells. Pharmacokinetic studies with mice showed that the oral bioavailability of bis(POC)-PMPA (calculated from the curves of the concentration of free PMPA in plasma) was 20%. Neither bis(POC)-PMPA nor mono(POC)-PMPA could be recovered in plasma, suggesting the efficient release of the active drug PMPA after oral administration of bis(POC)-PMPA. Severe combined immunodeficient (SCID) mice infected with Moloney murine sarcoma virus (MSV) and treated orally with bis(POC)-PMPA for 5 or 10 days (dosages, 50, 100, or 200 mg of PMPA equivalent per kg of body weight per day) showed a significant delay in MSV-induced tumor appearance and tumor-associated death. The antiviral efficacy of oral bis(POC)-PMPA was related to the dosage and treatment period and was not significantly different from that of subcutaneous PMPA given at an equivalent dose. The favorable pharmacokinetic profile, marked antiviral efficacy, and low toxicity make bis(POC)-PMPA an attractive oral prodrug of PMPA that should be further pursued in clinical studies with patients infected with human immunodeficiency virus or hepatitis B virus.
Figures
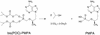

Similar articles
-
Inhibition of intestinal metabolism of the antiviral ester prodrug bis(POC)-PMPA by nature-identical fruit extracts as a strategy to enhance its oral absorption: an in vitro study.Pharm Res. 1999 Jul;16(7):1035-40. doi: 10.1023/a:1018931631912. Pharm Res. 1999. PMID: 10450927
-
Antiretroviral activity and pharmacokinetics in mice of oral bis(pivaloyloxymethyl)-9-(2-phosphonylmethoxyethyl)adenine, the bis(pivaloyloxymethyl) ester prodrug of 9-(2-phosphonylmethoxyethyl)adenine.Antimicrob Agents Chemother. 1996 Jan;40(1):22-8. doi: 10.1128/AAC.40.1.22. Antimicrob Agents Chemother. 1996. PMID: 8787873 Free PMC article.
-
Metabolism and pharmacokinetics of novel oral prodrugs of 9-[(R)-2-(phosphonomethoxy)propyl]adenine (PMPA) in dogs.Pharm Res. 1997 Dec;14(12):1824-9. doi: 10.1023/a:1012108719462. Pharm Res. 1997. PMID: 9453075
-
Tenofovir disoproxil fumarate: a nucleotide reverse transcriptase inhibitor for the treatment of HIV infection.Clin Ther. 2002 Oct;24(10):1515-48. doi: 10.1016/s0149-2918(02)80058-3. Clin Ther. 2002. PMID: 12462284 Review.
-
Acyclic nucleoside phosphonates in the chemotherapy of DNA virus and retrovirus infections.Intervirology. 1997;40(5-6):295-303. doi: 10.1159/000150563. Intervirology. 1997. PMID: 9675635 Review.
Cited by
-
Prodrug strategies for improved efficacy of nucleoside antiviral inhibitors.Curr Opin HIV AIDS. 2013 Nov;8(6):556-64. doi: 10.1097/COH.0000000000000007. Curr Opin HIV AIDS. 2013. PMID: 24100876 Free PMC article. Review.
-
Selected Milestones in Antiviral Drug Development.Viruses. 2024 Jan 23;16(2):169. doi: 10.3390/v16020169. Viruses. 2024. PMID: 38399945 Free PMC article. Review.
-
Antiviral prodrugs - the development of successful prodrug strategies for antiviral chemotherapy.Br J Pharmacol. 2006 Jan;147(1):1-11. doi: 10.1038/sj.bjp.0706446. Br J Pharmacol. 2006. PMID: 16284630 Free PMC article. Review.
-
Tribute to John C. Martin at the Twentieth Anniversary of the Breakthrough of Tenofovir in the Treatment of HIV Infections.Viruses. 2021 Dec 2;13(12):2410. doi: 10.3390/v13122410. Viruses. 2021. PMID: 34960679 Free PMC article. Review.
-
Inhibition of intestinal metabolism of the antiviral ester prodrug bis(POC)-PMPA by nature-identical fruit extracts as a strategy to enhance its oral absorption: an in vitro study.Pharm Res. 1999 Jul;16(7):1035-40. doi: 10.1023/a:1018931631912. Pharm Res. 1999. PMID: 10450927
References
-
- Annaert P, Kinget R, Naesens L, De Clercq E, Augustijns P. Transport, uptake and metabolism of the bis(pivaloyloxymethyl)-ester prodrug of 9-(2-phosphonylmethoxyethyl)adenine in an in vitro cell culture system of the intestinal mucosa (Caco-2) Pharm Res. 1997;14:492–496. - PubMed
-
- Arimilli M N, Kim C U, Dougherty J, Mulato A, Oliyai R, Shaw J P, Cundy K C, Bischofberger N. Synthesis, in vitro biological evaluation, and oral bioavailability of 9-[2-phosphonylmethoxy)propyl]adenine (PMPA) prodrugs. Antivir Chem Chemother. 1997;8:557–564.
-
- Balzarini J, Holy A, Jindrich J, Naesens L, Snoeck R, Schols D, De Clercq E. Differential antiherpesvirus and antiretrovirus effects of the (S) and (R) enantiomers of acyclic nucleoside phosphonates: potent and selective in vitro and in vivo antiretrovirus activities of (R)-9-(2-phosphonomethoxypropyl)-2,6-diaminopurine. Antimicrob Agents Chemother. 1993;37:332–338. - PMC - PubMed
-
- Barditch-Crovo P, Deeks S, Kahn J, Redpath I, Smith A, Hwang F, Hellmann N, Cundy K, Rooney J, Lietman P. PMPA: safety, pharmacokinetics, and antiretroviral activity when administered as a single dose and for seven consecutive days to patients with HIV infection. Presented at the 10th International Conference on Antiviral Research. 1997.
-
- Barditch-Crovo P, Toole J, Hendrix C W, Cundy K C, Ebeling D, Jaffe H S, Lietman P S. Anti-human immunodeficiency virus (HIV) activity, safety, and pharmacokinetics of adefovir dipivoxyl (9-[2-(bis(pivaloyloxymethyl)phosphonylmethoxyethyl]adenine) in HIV-infected patients. J Infect Dis. 1997;176:406–413. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

