Pharmacokinetic evaluation of amphotericin B in lung tissue: lung lymph distribution after intravenous injection and airspace distribution after aerosolization and inhalation of amphotericin B
- PMID: 9660990
- PMCID: PMC105652
- DOI: 10.1128/AAC.42.7.1597
Pharmacokinetic evaluation of amphotericin B in lung tissue: lung lymph distribution after intravenous injection and airspace distribution after aerosolization and inhalation of amphotericin B
Abstract
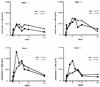
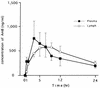
We have studied the pharmacokinetics of amphotericin B (AmB) in lung lymph circulation and bronchial-wash fluid after intravenous infusion and inhalation, respectively. For two experiments with awake sheep, we used lung lymph fistulas and tracheotomy. In experiment 1, AmB concentrations in plasma and lung lymph after intravenous infusion of AmB (1 mg/kg of body weight) over 1.5 h were measured. The mean peak in plasma level was 756.0 +/- 188.8 ng/ml at 3 h after the start of infusion, and the level then decreased gradually to 194.8 +/- 28.9 ng/ml at 24 h. The stable and maximal levels in lung lymph last 5 to 9 h after the start of AmB infusion. The concentrations in lung lymph after 9 h were slightly higher than those in plasma. Thus, the lung lymph-to-plasma ratio of AmB concentrations increased gradually during infusion, and the ratio was more than 1.0 after the end of infusion, suggesting that AmB could be easily moved from plasma to pulmonary interstitium and/or lung lymph circulation. In another experiment, 5 or 30 mg of aerosol AmB was inhaled, and the concentration of AmB in the bronchial-wash fluid was determined by bronchoalveolar lavage. The peak AmB concentration in the fluid was observed at 0.5 h. After that, AmB was slowly eliminated over 24 h. The area under the concentration-time curve for 30 mg of inhaled AmB was higher than that for 5 mg, but maximum concentrations of AmB in serum for 5 and 30 mg were almost similar. These observations identify the pharmacokinetic characteristics of AmB in the lung and may provide a new insight into the strategy for clinical treatment of fungal pneumonia.
Figures
Similar articles
-
Pharmacokinetics of aerosol amphotericin B in rats.Antimicrob Agents Chemother. 1990 Jan;34(1):29-32. doi: 10.1128/AAC.34.1.29. Antimicrob Agents Chemother. 1990. PMID: 2327759 Free PMC article.
-
Disposition of aerosolized liposomal amphotericin B.J Pharm Sci. 1997 Sep;86(9):1066-9. doi: 10.1021/js9604218. J Pharm Sci. 1997. PMID: 9294824
-
Lung tissue distribution after intravenous administration of grepafloxacin: comparative study with levofloxacin.Jpn J Pharmacol. 2002 Jan;88(1):63-8. doi: 10.1254/jjp.88.63. Jpn J Pharmacol. 2002. PMID: 11855679
-
A review on the clinical use of inhaled amphotericin B.J Aerosol Med Pulm Drug Deliv. 2009 Sep;22(3):213-27. doi: 10.1089/jamp.2008.0715. J Aerosol Med Pulm Drug Deliv. 2009. PMID: 19466905 Review.
-
Pharmaceutical design of the liposomal antimicrobial agents for infectious disease.Curr Pharm Des. 2002;8(6):441-54. doi: 10.2174/1381612023395853. Curr Pharm Des. 2002. PMID: 12069381 Review.
Cited by
-
Compartmentalized intrapulmonary pharmacokinetics of amphotericin B and its lipid formulations.Antimicrob Agents Chemother. 2006 Oct;50(10):3418-23. doi: 10.1128/AAC.00241-06. Antimicrob Agents Chemother. 2006. PMID: 17005824 Free PMC article.
-
Pentamidine is active in vitro against Fusarium species.Antimicrob Agents Chemother. 2003 Oct;47(10):3252-9. doi: 10.1128/AAC.47.10.3252-3259.2003. Antimicrob Agents Chemother. 2003. PMID: 14506038 Free PMC article.
-
Biodistribution and histopathology studies of amphotericin B sodium deoxycholate sulfate formulation following intratracheal instillation in rat models.Drug Deliv Transl Res. 2020 Feb;10(1):59-69. doi: 10.1007/s13346-019-00662-x. Drug Deliv Transl Res. 2020. PMID: 31368043
-
Aerosolized amphotericin B lipid complex as adjunctive treatment for fungal lung infection in patients with cancer-related immunosuppression and recipients of hematopoietic stem cell transplantation.Pharmacotherapy. 2013 Oct;33(10):1035-43. doi: 10.1002/phar.1309. Epub 2013 Jun 19. Pharmacotherapy. 2013. PMID: 23784915 Free PMC article.
-
Efficacy of nebulized liposomal amphotericin B in treatment of experimental pulmonary aspergillosis.Antimicrob Agents Chemother. 2005 Jul;49(7):3028-30. doi: 10.1128/AAC.49.7.3028-3030.2005. Antimicrob Agents Chemother. 2005. PMID: 15980392 Free PMC article.
References
-
- Bennett J E. Chemotherapy of systemic mycoses (first of two parts) N Engl J Med. 1974;290:30–32. - PubMed
-
- Bindschadler D D, Bennett J E. A pharmacologic guide to the clinical use of amphotericin B. J Infect Dis. 1969;120:427–436. - PubMed
-
- Block E R, Bennett J E, Livoti L G, Klein W J, MacGregor R R, Henderson L. Flucytosine and amphotericin B; hemodialysis effects on the plasma concentration and clearance. Ann Intern Med. 1974;80:613–617. - PubMed
-
- Brajtburg J, Elberg S, Bolard J, Kobayashi G S, Levi R A, Ostlund R E, Schlessinger D, Medoff G. Interaction of plasma proteins and lipoproteins with amphotericin B. J Infect Dis. 1984;149:986–997. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical