Cefepime-aztreonam: a unique double beta-lactam combination for Pseudomonas aeruginosa
- PMID: 9660993
- PMCID: PMC105655
- DOI: 10.1128/AAC.42.7.1610
Cefepime-aztreonam: a unique double beta-lactam combination for Pseudomonas aeruginosa
Abstract
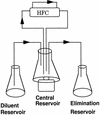
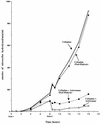
An in vitro pharmacokinetic model was used to determine if aztreonam could enhance the pharmacodynamics of cefepime or ceftazidime against an isogenic panel of Pseudomonas aeruginosa 164, including wild-type (WT), partially derepressed (PD), and fully derepressed (FD) phenotypes. Logarithmic-phase cultures were exposed to peak concentrations achieved in serum with 1- or 2-g intravenous doses, elimination pharmacokinetics were simulated, and viable bacterial counts were measured over three 8-h dosing intervals. In studies with cefepime and cefepime-aztreonam against the PD strain, samples were also filter sterilized, assayed for active cefepime, and assayed for nitrocefin hydrolysis activity before and after overnight dialysis. Against WT strains, the cefepime-aztreonam combination was the most active regimen, but viable counts at 24 h were only 1 log below those in cefepime-treated cultures. Against PD and FD strains, the antibacterial activity of cefepime-aztreonam was significantly enhanced over that of each drug alone, with 3.5 logs of killing by 24 h. Hydrolysis and bioassay studies demonstrated that aztreonam was inhibiting the extracellular cephalosporinase that had accumulated and was thus protecting cefepime in the extracellular environment. In contrast to cefepime-aztreonam, the pharmacodynamics of ceftazidime-aztreonam were not enhanced over those of aztreonam alone. Further pharmacodynamic studies with five other P. aeruginosa strains producing increased levels of cephalosporinase demonstrated that the enhanced pharmacodynamics of cefepime-aztreonam were not unique to the isogenic panel. The results of these studies demonstrate that aztreonam can enhance the antibacterial activity of cefepime against derepressed mutants of P. aeruginosa producing increased levels of cephalosporinase. This positive interaction appears to be due in part to the ability of aztreonam to protect cefepime from extracellular cephalosporinase inactivation. Clinical evaluation of this combination is warranted.
Figures

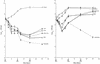
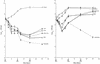

Similar articles
-
[In vitro study of antibacterial activity of cefepime (Axepim) against gram negative bacteria: comparison with cephalosporins and other beta-lactams].Pathol Biol (Paris). 1996 Feb;44(2):132-7. Pathol Biol (Paris). 1996. PMID: 8761598 French.
-
Pharmacodynamics of once-daily amikacin in various combinations with cefepime, aztreonam, and ceftazidime against Pseudomonas aeruginosa in an in vitro infection model.Antimicrob Agents Chemother. 1992 Dec;36(12):2741-6. doi: 10.1128/AAC.36.12.2741. Antimicrob Agents Chemother. 1992. PMID: 1482142 Free PMC article.
-
Evaluation of several dosing regimens of cefepime, with various simulations of renal function, against clinical isolates of Pseudomonas aeruginosa in a pharmacodynamic infection model.Antimicrob Agents Chemother. 1999 Jan;43(1):129-33. doi: 10.1128/AAC.43.1.129. Antimicrob Agents Chemother. 1999. PMID: 9869577 Free PMC article.
-
Cefiderocol: A Siderophore Cephalosporin with Activity Against Carbapenem-Resistant and Multidrug-Resistant Gram-Negative Bacilli.Drugs. 2019 Feb;79(3):271-289. doi: 10.1007/s40265-019-1055-2. Drugs. 2019. PMID: 30712199 Review.
-
Pharmacodynamic considerations in the treatment of moderate to severe pseudomonal infections with cefepime.J Antimicrob Chemother. 2002 Mar;49(3):445-53. doi: 10.1093/jac/49.3.445. J Antimicrob Chemother. 2002. PMID: 11864944 Review.
Cited by
-
Age of Antibiotic Resistance in MDR/XDR Clinical Pathogen of Pseudomonas aeruginosa.Pharmaceuticals (Basel). 2023 Aug 30;16(9):1230. doi: 10.3390/ph16091230. Pharmaceuticals (Basel). 2023. PMID: 37765038 Free PMC article. Review.
-
Antibiotic selection in the treatment of acute invasive infections by Pseudomonas aeruginosa: Guidelines by the Spanish Society of Chemotherapy.Rev Esp Quimioter. 2018 Feb;31(1):78-100. Epub 2018 Feb 23. Rev Esp Quimioter. 2018. PMID: 29480677 Free PMC article. Review.
-
In vitro activities of antibiotic combinations against clincal isolates of Pseudomonas aeruginosa.Kaohsiung J Med Sci. 2004 Jun;20(6):261-7. doi: 10.1016/s1607-551x(09)70116-0. Kaohsiung J Med Sci. 2004. PMID: 15253466 Free PMC article.
-
Modeling in vivo pharmacokinetics and pharmacodynamics of moxifloxacin therapy for Mycobacterium tuberculosis infection by using a novel cartridge system.Antimicrob Agents Chemother. 2005 Feb;49(2):853-6. doi: 10.1128/AAC.49.2.853-856.2005. Antimicrob Agents Chemother. 2005. PMID: 15673788 Free PMC article.
-
Dual β-lactam combination therapy for multi-drug resistant Pseudomonas aeruginosa infection: enhanced efficacy in vivo and comparison with monotherapies of penicillin-binding protein inhibition.Sci Rep. 2019 Jun 24;9(1):9098. doi: 10.1038/s41598-019-45550-z. Sci Rep. 2019. PMID: 31235728 Free PMC article.
References
-
- Akova M, Yang Y, Livermore D M. Interactions of tazobactam and clavulanate with inducibly- and constitutively-expressed class I β-lactamase. J Antimicrob Chemother. 1990;25:199–208. - PubMed
-
- Blaser, J., B. B. Stone, and S. H. Zinner. 1985. Two compartment kinetic model with multiple artificial capillary units. J. Antimicrob. Chemother. 15(Suppl. A):131–137. - PubMed
-
- Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

