Administration of liposomal agents and blood clearance capacity of the mononuclear phagocyte system
- PMID: 9661003
- PMCID: PMC105665
- DOI: 10.1128/AAC.42.7.1677
Administration of liposomal agents and blood clearance capacity of the mononuclear phagocyte system
Abstract
As liposomes are cleared from the circulation to a substantial extent by the phagocytic cells of the mononuclear phagocyte system (MPS), there is a question whether administration of liposome-based therapeutic agents interferes with clearance of infectious organisms by the MPS from blood. In the present study, at first the effect of administration of three types of empty liposomes (devoid of drug), differing in blood residence time, on carbon clearance and bacterial clearance from blood was studied with mice. Classical liposomes (LIP A) and placebo liposomes with lipid composition as in AmBisome (LIP B) or as in Doxil (LIP C) were used. Liposomes were administered intravenously as a single dose. Second, the effect of multiple-dose administration of AmBisome on bacterial blood clearance was studied with rats. AmBisome was administered with two different dosage schedules. The blood clearance capacity of the MPS was monitored at different time points after the last liposome injection. It was shown that the carbon blood clearance capacity of the MPS was impaired only at a high lipid dose of empty classical liposomes. The bacterial blood clearance capacity was never impaired, not even after prolonged treatment with AmBisome administered in a clinically relevant regimen.
Figures
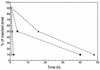

 ). ∗, P ≤ 0.001 versus
buffer-treated mice.
). ∗, P ≤ 0.001 versus
buffer-treated mice.



Similar articles
-
Doxorubicin entrapped in sterically stabilized liposomes: effects on bacterial blood clearance capacity of the mononuclear phagocyte system.Clin Cancer Res. 1998 Jan;4(1):111-5. Clin Cancer Res. 1998. PMID: 9516959
-
Effects on the murine mononuclear phagocyte system of chronic administration of liposomes containing cytotoxic drug or lipid A compared with empty liposomes.Can J Physiol Pharmacol. 1987 Feb;65(2):185-90. doi: 10.1139/y87-035. Can J Physiol Pharmacol. 1987. PMID: 2436731
-
Liposomal doxorubicin-induced toxicity: depletion and impairment of phagocytic activity of liver macrophages.Int J Cancer. 1995 May 29;61(5):716-21. doi: 10.1002/ijc.2910610520. Int J Cancer. 1995. PMID: 7768646
-
Liposome clearance.Biosci Rep. 2002 Apr;22(2):197-224. doi: 10.1023/a:1020134521778. Biosci Rep. 2002. PMID: 12428901 Review.
-
Liposomes in the treatment of infections.J Drug Target. 1994;2(5):363-71. doi: 10.3109/10611869408996811. J Drug Target. 1994. PMID: 7704480 Review.
Cited by
-
Clinical Pharmacokinetics, Pharmacodynamics, Safety and Efficacy of Liposomal Amphotericin B.Clin Infect Dis. 2019 May 2;68(Suppl 4):S260-S274. doi: 10.1093/cid/ciz076. Clin Infect Dis. 2019. PMID: 31222253 Free PMC article. Review.
-
Liposomes as Antibiotic Delivery Systems: A Promising Nanotechnological Strategy against Antimicrobial Resistance.Molecules. 2021 Apr 2;26(7):2047. doi: 10.3390/molecules26072047. Molecules. 2021. PMID: 33918529 Free PMC article. Review.
-
Ciprofloxacin in polyethylene glycol-coated liposomes: efficacy in rat models of acute or chronic Pseudomonas aeruginosa infection.Antimicrob Agents Chemother. 2002 Aug;46(8):2575-81. doi: 10.1128/AAC.46.8.2575-2581.2002. Antimicrob Agents Chemother. 2002. PMID: 12121935 Free PMC article.
-
Pharmacodynamics and Biodistribution of Single-Dose Liposomal Amphotericin B at Different Stages of Experimental Visceral Leishmaniasis.Antimicrob Agents Chemother. 2017 Aug 24;61(9):e00497-17. doi: 10.1128/AAC.00497-17. Print 2017 Sep. Antimicrob Agents Chemother. 2017. PMID: 28630200 Free PMC article.
-
Preclinical Safety, Tolerability, Pharmacokinetics, Pharmacodynamics, and Antifungal Activity of Liposomal Amphotericin B.Clin Infect Dis. 2019 May 2;68(Suppl 4):S244-S259. doi: 10.1093/cid/ciz064. Clin Infect Dis. 2019. PMID: 31222254 Free PMC article. Review.
References
-
- Allen T M, Murray L, MacKeigan S, Shah M. Chronic liposome administration in mice: effects on reticuloendothelial function and tissue distribution. J Pharmacol Exp Ther. 1984;229:267–275. - PubMed
-
- Ames B N, Dubin D T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960;235:769–775. - PubMed
-
- Arning M, Kliche K O, Heer-Sonderhoff A H, Wehmeier A. Infusion related toxicity of three different amphotericin B formulations and its relation to cytokine plasma levels. Mycoses. 1995;38:459–465. - PubMed
-
- Bakker-Woudenberg I A J M, Lokerse A F, Roerdink F H, Regts J, Michel M F. Free versus liposome-entrapped ampicillin in treatment of infection due to Listeria monocytogenesin normal and athymic (nude) mice. J Infect Dis. 1985;151:917–924. - PubMed
-
- Bakker-Woudenberg I A J M, ten Kate M T, Stearne-Cullen L E T, Woodle M C. Efficacy of gentamicin or ceftazidime entrapped in liposomes with prolonged blood circulation and enhanced localization in Klebsiella pneumoniae-infected lung tissue. J Infect Dis. 1995;171:938–947. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

