Metabolism of 2',3'-dideoxy-2',3'-didehydro-beta-L(-)-5-fluorocytidine and its activity in combination with clinically approved anti-human immunodeficiency virus beta-D(+) nucleoside analogs in vitro
- PMID: 9661024
- PMCID: PMC105686
- DOI: 10.1128/AAC.42.7.1799
Metabolism of 2',3'-dideoxy-2',3'-didehydro-beta-L(-)-5-fluorocytidine and its activity in combination with clinically approved anti-human immunodeficiency virus beta-D(+) nucleoside analogs in vitro
Abstract
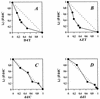
2',3'-Dideoxy-2',3'-didehydro-beta-L(-)-5-fluorocytidine [L(-)Fd4C] has been reported to be a potent inhibitor of the human immunodeficiency virus (HIV) in cell culture. In the present study the antiviral activity of this compound in two-drug combinations and its intracellular metabolism are addressed. The two-drug combination of L(-)Fd4C plus 2',3'-didehydro-2'-3'-dideoxythymidine (D4T, or stavudine) or 3'-azido-3'-deoxythymidine (AZT, or zidovudine) synergistically inhibited replication of HIV in vitro. Additive antiviral activity was observed with L(-)Fd4C in combination with 2',3'-dideoxycytidine (ddC, or zalcitabine) or 2',3'-dideoxyinosine (ddI, or didanosine). This beta-L(-) nucleoside analog has no activity against mitochondrial DNA synthesis at concentrations up to 10 microM. As we previously reported for other beta-L(-) nucleoside analogs, L(-)Fd4C could protect against mitochondrial toxicity associated with D4T, ddC, and ddI. Metabolism studies showed that this drug is converted intracellularly to its mono-, di-, and triphosphate metabolites. The enzyme responsible for monophosphate formation was identified as cytoplasmic deoxycytidine kinase, and the K(m) is 100 microM. L(-)Fd4C was not recognized in vitro by human mitochondrial deoxypyrimidine nucleoside kinase. Also, L(-)Fd4C was not a substrate for deoxycytidine deaminase. L(-)Fd4C 5'-triphosphate served as an alternative substrate to dCTP for incorporation into DNA by HIV reverse transcriptase. The favorable anti-HIV activity and protection from mitochondrial toxicity by L(-)Fd4C in two-drug combinations favors the further development of L(-)Fd4C as an anti-HIV agent.
Figures


Similar articles
-
Characterization of the antiviral effect of 2',3'-dideoxy-2', 3'-didehydro-beta-L-5-fluorocytidine in the duck hepatitis B virus infection model.Antimicrob Agents Chemother. 2000 Jan;44(1):111-22. doi: 10.1128/AAC.44.1.111-122.2000. Antimicrob Agents Chemother. 2000. PMID: 10602731 Free PMC article.
-
Anti-hepatitis B virus activity and metabolism of 2',3'-dideoxy-2',3'-didehydro-beta-L(-)-5-fluorocytidine.Antimicrob Agents Chemother. 1998 Jul;42(7):1805-10. doi: 10.1128/AAC.42.7.1805. Antimicrob Agents Chemother. 1998. PMID: 9661025 Free PMC article.
-
Effect of nucleoside analogs on neurite regeneration and mitochondrial DNA synthesis in PC-12 cells.J Pharmacol Exp Ther. 1997 Mar;280(3):1228-34. J Pharmacol Exp Ther. 1997. PMID: 9067308
-
Comparative evaluation of L-Fd4C and related nucleoside analogs as promising antiviral agents.Curr Med Chem. 2002 May;9(9):899-912. doi: 10.2174/0929867024606696. Curr Med Chem. 2002. PMID: 11966452 Review.
-
Perspectives on the molecular mechanism of inhibition and toxicity of nucleoside analogs that target HIV-1 reverse transcriptase.Biochim Biophys Acta. 2002 Jul 18;1587(2-3):296-9. doi: 10.1016/s0925-4439(02)00092-3. Biochim Biophys Acta. 2002. PMID: 12084471 Review.
Cited by
-
HIV-1 Reverse Transcriptase Still Remains a New Drug Target: Structure, Function, Classical Inhibitors, and New Inhibitors with Innovative Mechanisms of Actions.Mol Biol Int. 2012;2012:586401. doi: 10.1155/2012/586401. Epub 2012 Jun 20. Mol Biol Int. 2012. PMID: 22778958 Free PMC article.
-
Anti-Epstein-Barr virus (EBV) activity of beta-L-5-iododioxolane uracil is dependent on EBV thymidine kinase.Antimicrob Agents Chemother. 2000 Dec;44(12):3278-84. doi: 10.1128/AAC.44.12.3278-3284.2000. Antimicrob Agents Chemother. 2000. PMID: 11083627 Free PMC article.
-
Comparative study of the persistence of anti-HIV activity of deoxynucleoside HIV reverse transcriptase inhibitors after removal from culture.AIDS Res Ther. 2009 Apr 22;6:5. doi: 10.1186/1742-6405-6-5. AIDS Res Ther. 2009. PMID: 19386130 Free PMC article.
-
Mitochondrial toxicity and HIV therapy.Sex Transm Infect. 2001 Jun;77(3):158-73. doi: 10.1136/sti.77.3.158. Sex Transm Infect. 2001. PMID: 11402222 Free PMC article. Review.
-
Characterization of the antiviral effect of 2',3'-dideoxy-2', 3'-didehydro-beta-L-5-fluorocytidine in the duck hepatitis B virus infection model.Antimicrob Agents Chemother. 2000 Jan;44(1):111-22. doi: 10.1128/AAC.44.1.111-122.2000. Antimicrob Agents Chemother. 2000. PMID: 10602731 Free PMC article.
References
-
- Bridges E G, Dutschman G E, Gullen E A, Cheng Y-C. Favorable interaction of β-l(−) nucleoside analogues with clinically approved anti-HIV nucleoside analogues for the treatment of human immunodeficiency virus. Biochem Pharmacol. 1996;51:731–736. - PubMed
-
- Bridges E G, Jiang Z L, Cheng Y C. Identification of a novel mitochondrial dNTP carrier and its interaction with anti-HIV nucleoside analogs. Proc Am Assoc Cancer Res. 1997;38:414.
-
- Chang C-N, Doong S-L, Zhou J H, Beach J W, Jeong L S, Chu C K, Tsai C-H, Liotta D C, Schinazi R F, Cheng Y-C. Deoxycytidine deaminase-resistant stereoisomer is the active form of (±)-2′,3′-dideoxy-3′-thiacytidine in the inhibition of hepatitis B virus replication. J Biol Chem. 1992;267:13938–13942. - PubMed
-
- Chang C N, Skalski V, Zhou J H, Cheng Y-C. Biochemical pharmacology of (+) and (−)-2′,3′-dideoxy-3′-thiacytidine as anti-hepatitis B virus agents. J Biol Chem. 1992;267:22414–22420. - PubMed
-
- Chen C-H, Vazquez-Padua M, Cheng Y-C. The effect of anti-HIV nucleoside analogs on mitochondrial DNA and its implication for delayed toxicity. Mol Pharmacol. 1991;39:625–628. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

