Enhanced bioavailability of itraconazole in hydroxypropyl-beta-cyclodextrin solution versus capsules in healthy volunteers
- PMID: 9661037
- PMCID: PMC105699
- DOI: 10.1128/AAC.42.7.1862
Enhanced bioavailability of itraconazole in hydroxypropyl-beta-cyclodextrin solution versus capsules in healthy volunteers
Abstract
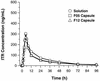
The bioavailabilities and bioequivalences of single 200-mg doses of itraconazole solution and two capsule formulations were evaluated in a crossover study of 30 male volunteers. The two capsule formulations were bioequivalent. The bioavailabilities of the solutions itraconazole and hydroxyitraconazole were 30 to 33% and 35 to 37% greater, respectively, than those of either capsule. However, the maximum concentrations of the drug in plasma (Cmax), the times to Cmax, and the terminal half-lives were comparable for all three formulations. These data indicate that the bioavailabilities of itraconazole and hydroxyitraconazole are enhanced when administered as an oral solution instead of capsules.
Figures
References
-
- Barchiesi F, Colombo A L, McGough D A, Fothergill A W, Rinaldi M G. In vitro activity of itraconazole against fluconazole-susceptible and resistant Candida albicans isolates from oral cavities of patients infected with human immunodeficiency virus. Antimicrob Agents Chemother. 1994;38:1530–1533. - PMC - PubMed
-
- Barone J A, Moskovitz B L, Guarnieri J, Hassell A E, Colaizzi J L, Bierman R H, Jessen L. Food interaction and steady-state pharmacokinetics of itraconazole (ITR) oral solution in healthy volunteers. Pharmacotherapy. 1997;17:195. . (Abstract 76.) - PubMed
-
- Blatchford N R. Treatment of oral candidosis with itraconazole: a review. J Am Acad Dermatol. 1990;23:565–567. - PubMed
-
- Bradford C R, Prentice K A G, Warnock D W, Copplestone J A. Comparison of the multiple dose pharmacokinetics of two formulations of itraconazole during remission induction for acute myeloblastic leukaemia. J Antimicrob Chemother. 1991;28:555–560. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources