Evolution of C4 photosynthesis in flaveria species. Isoforms Of nadp-malic enzyme
- PMID: 9662516
- PMCID: PMC34928
- DOI: 10.1104/pp.117.3.733
Evolution of C4 photosynthesis in flaveria species. Isoforms Of nadp-malic enzyme
Abstract
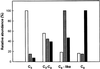
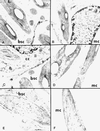
NADP-malic enzyme (NADP-ME, EC 1.1.1.40), a key enzyme in C4 photosynthesis, provides CO2 to the bundle-sheath chloroplasts, where it is fixed by ribulose-1,5-bisphosphate carboxylase/oxygenase. We characterized the isoform pattern of NADP-ME in different photosynthetic species of Flaveria (C3, C3-C4 intermediate, C4-like, C4) based on sucrose density gradient centrifugation and isoelectric focusing of the native protein, western-blot analysis of the denatured protein, and in situ immunolocalization with antibody against the 62-kD C4 isoform of maize. A 72-kD isoform, present to varying degrees in all species examined, is predominant in leaves of C3 Flaveria spp. and is also present in stem and root tissue. By immunolabeling, NADP-ME was found to be mostly localized in the upper palisade mesophyll chloroplasts of C3 photosynthetic tissue. Two other isoforms of the enzyme, with molecular masses of 62 and 64 kD, occur in leaves of certain intermediates having C4 cycle activity. The 62-kD isoform, which is the predominant highly active form in the C4 species, is localized in bundle-sheath chloroplasts. Among Flaveria spp. there is a 72-kD constitutive form, a 64-kD form that may have appeared during evolution of C4 metabolism, and a 62-kD form that is necessary for the complete functioning of C4 photosynthesis.
Figures




References
-
- Borsch D, Westhoff P. Primary structure of NADP-dependent malic enzyme in the dicotyledonous Flaveria trinervia. FEBS Lett. 1990;273:111–115. - PubMed
-
- Burnette WN. “Western blotting”: electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981;112:195–203. - PubMed
-
- Casati P, Spampinato CP, Andreo CS. Characteristics and physiological function of NADP-malic enzyme from wheat. Plant Cell Physiol. 1997;38:928–934.
LinkOut - more resources
Full Text Sources
Miscellaneous

