Developmental expression and substrate specificities of alfalfa caffeic acid 3-O-methyltransferase and caffeoyl coenzyme A 3-O-methyltransferase in relation to lignification
- PMID: 9662519
- PMCID: PMC34931
- DOI: 10.1104/pp.117.3.761
Developmental expression and substrate specificities of alfalfa caffeic acid 3-O-methyltransferase and caffeoyl coenzyme A 3-O-methyltransferase in relation to lignification
Abstract
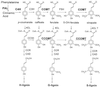
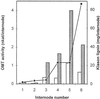
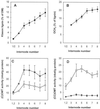
The biosynthesis of monolignols can potentially occur via two parallel pathways involving free acids or their coenzyme A (CoA) esters. Caffeic acid 3-O-methyltransferase (COMT) and caffeoyl CoA 3-O-methyltransferase (CCOMT) catalyze functionally identical reactions in these two pathways, resulting in the formation of mono- or dimethoxylated lignin precursors. The activities of the two enzymes increase from the first to the sixth internode in stems of alfalfa (Medicago sativa L.), preceding the deposition of lignin. Alfalfa CCOMT is highly similar at the amino acid sequence level to the CCOMT from parsley, although it contains a six-amino acid insertion near the N terminus. Transcripts encoding both COMT and CCOMT are primarily localized to vascular tissue in alfalfa stems. Alfalfa CCOMT expressed in Escherichia coli catalyzes O-methylation of caffeoyl and 5-hydroxyferuloyl CoA, with preference for caffeoyl CoA. It has low activity against the free acids. COMT expressed in E. coli is active against both caffeic and 5-hydroxyferulic acids, with preference for the latter compound. Surprisingly, very little extractable O-methyltransferase activity versus 5-hydroxyferuloyl CoA is present in alfalfa stem internodes, in which relative O-methyltransferase activity against 5-hy-droxyferulic acid increases with increasing maturity, correlating with increased lignin methoxyl content.
Figures



References
-
- Akin DE. Histological and physical factors affecting digestibility of forages. Agron J. 1989;81:17–25.
-
- Albrecht KA, Wedin WF, Buxton DR. Cell-wall composition and digestibility of alfalfa stems and leaves. Crop Sci. 1987;27:735–741.
-
- Atanassova R, Favet N, Martz F, Chabbert B, Tollier M-T, Monties B, Fritig B, Legrand M. Altered lignin composition in transgenic tobacco expressing O-methyltransferase sequences in sense and antisense orientation. Plant J. 1995;8:465–477.
-
- Baker SM. Rapid methoxyl analysis of lignins using gas chromatography. Holzforschung. 1996;50:573–574.
-
- Banerjee SK, Manolopoulo M, Pepper JM. The synthesis of lignin model substances: 5-hydroxyvanillin and 5-hydroxyacetoguaiacone. Can J Chem. 1962;40:2175–2177.
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

