Biosynthesis of the monoterpenes limonene and carvone in the fruit of caraway. I. Demonstration Of enzyme activities and their changes with development
- PMID: 9662532
- PMCID: PMC34944
- DOI: 10.1104/pp.117.3.901
Biosynthesis of the monoterpenes limonene and carvone in the fruit of caraway. I. Demonstration Of enzyme activities and their changes with development
Abstract
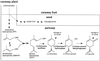
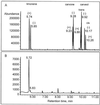
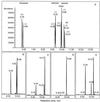
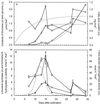
The biosynthesis of the monoterpenes limonene and carvone in the fruit of caraway (Carum carvi L.) proceeds from geranyl diphosphate via a three-step pathway. First, geranyl diphosphate is cyclized to (+)-limonene by a monoterpene synthase. Second, this intermediate is stored in the essential oil ducts without further metabolism or is converted by limonene-6-hydroxylase to (+)-trans-carveol. Third, (+)-trans-carveol is oxidized by a dehydrogenase to (+)-carvone. To investigate the regulation of monoterpene formation in caraway, we measured the time course of limonene and carvone accumulation during fruit development and compared it with monoterpene biosynthesis from [U-14C]Suc and the changes in the activities of the three enzymes. The activities of the enzymes explain the profiles of monoterpene accumulation quite well, with limonene-6-hydroxylase playing a pivotal role in controlling the nature of the end product. In the youngest stages, when limonene-6-hydroxylase is undetectable, only limonene was accumulating in appreciable levels. The appearance of limonene-6-hydroxylase correlates closely with the onset of carvone accumulation. At later stages of fruit development, the activities of all three enzymes declined to low levels. Although this correlates closely with a decrease in monoterpene accumulation, the latter may also be the result of competition with other pathways for substrate.
Figures
References
-
- Alonso WR, Croteau R. Prenyltransferases and cyclases. In: Lea PJ, editor. Methods in Plant Biochemistry, Vol 9: Enzymes of Secondary Metabolism. London: Academic Press; 1993. pp. 239–260.
-
- Banthorpe DV, Charlwood BV. The terpenoids. In: Bell EA, Charlwood BV, editors. Encyclopedia of Plant Physiology, New Series, Vol 8: Secondary Plant Products. Berlin: Springer-Verlag; 1980. pp. 185–220.
-
- Bolwell GP, Bozak K, Zimmerlin A. Plant cytochrome P450. Phytochemistry. 1994;37:1491–1506. - PubMed
-
- Bosabalidis AM. Ontogenesis, ultrastructure and morphometry of the petiole oil ducts of celery (Apium graveolens L.) Flavour Fragrance J. 1996;11:269–274.
-
- Bouwmeester HJ, Davies JAR, Smid HG, Welten RSA. Physiological limitations to carvone yield in caraway (Carum carvi L.) Ind Crops Prod. 1995a;4:39–51.
LinkOut - more resources
Full Text Sources

