Potent inhibition of ribulose-bisphosphate carboxylase by an oxidized impurity in ribulose-1,5-bisphosphate
- PMID: 9662549
- PMCID: PMC34922
- DOI: 10.1104/pp.117.3.1059
Potent inhibition of ribulose-bisphosphate carboxylase by an oxidized impurity in ribulose-1,5-bisphosphate
Abstract
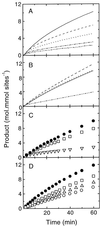
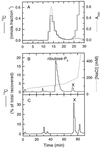
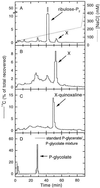
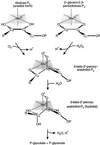
Oxidation of D-ribulose-1,5-bisphosphate (ribulose-P2) during synthesis and/or storage produces D-glycero-2,3-pentodiulose-1, 5-bisphosphate (pentodiulose-P2), a potent slow, tight-binding inhibitor of spinach (Spinacia oleracea L.) ribulose-P2 carboxylase/oxygenase (Rubisco). Differing degrees of contamination with pentodiulose-P2 caused the decline in Rubisco activity seen during Rubisco assay time courses to vary between different preparations of ribulose-P2. With some ribulose-P2 preparations, this compound can be the dominant cause of the decline, far exceeding the significance of the catalytic by-product, D-xylulose-1, 5-bisphosphate. Unlike xylulose-1,5-bisphosphate, pentodiulose-P2 did not appear to be a significant by-product of catalysis by wild-type Rubisco at saturating CO2 concentration. It was produced slowly during frozen storage of ribulose-P2, even at low pH, more rapidly in Rubisco assay buffers at room temperature, and particularly rapidly on deliberate oxidation of ribulose-P2 with Cu2+. Its formation was prevented by the exclusion of transition metals and O2. Pentodiulose-P2 was unstable and decayed to a variety of other less-inhibitory compounds, particularly in the presence of some buffers. However, it formed a tight, stable complex with carbamylated spinach Rubisco, which could be isolated by gel filtration, presumably because its structure mimics that of the enediol intermediate of Rubisco catalysis. Rubisco catalyzes the cleavage of pentodiulose-P2 by H2O2, producing P-glycolate.
Figures
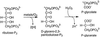

References
-
- Andrews TJ, Kane HJ. Pyruvate is a by-product of catalysis by ribulosebisphosphate carboxylase/oxygenase. J Biol Chem. 1991;266:9447–9452. - PubMed
-
- Andrews TJ, Lorimer GH (1987) Rubisco: structure, mechanisms, and prospects for improvement. In MD Hatch, NK Boardman, eds, The Biochemistry of Plants: A Comprehensive Treatise, Vol. 10, Photosynthesis. Academic Press, New York, pp 131–218
-
- Asada K. Production and action of active oxygen species in photosynthetic tissues. In: Foyer CH, Mullineaux PM, editors. Causes of Photooxidative Stress and Amelioration of Defense Systems in Plants. Boca Raton, FL: CRC Press; 1994. pp. 77–104.
-
- Badger MR, Andrews TJ, Canvin DT, Lorimer GH. Interaction of hydrogen peroxide with ribulose bisphosphate carboxylase-oxygenase. J Biol Chem. 1980;255:7870–7875. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

