Comparison of the effects of moclobemide and selegiline on tyramine-evoked mydriasis in man
- PMID: 9663810
- PMCID: PMC1873648
- DOI: 10.1046/j.1365-2125.1998.00729.x
Comparison of the effects of moclobemide and selegiline on tyramine-evoked mydriasis in man
Abstract
Aims: To examine the feasibility of using the human iris in vivo for the assessment of the interaction between tyramine and monoamine oxidase (MAO) inhibitors. To examine the relative roles of the two forms of MAO in terminating the response to sympathomimetic amines in the iris, by comparing the effects of single oral doses of moclobemide, a selective MAO-A inhibitor, and selegiline, a selective MAO-B inhibitor, on mydriatic responses to tyramine.
Methods: Twelve healthy male volunteers participated in three monthly sessions, each associated with ingestion of one capsule (moclobemide 450 mg, selegiline 10 mg, or placebo), according to a double-blind, balanced, cross-over design. Tyramine hydrochloride eye-drops (75 mM, 2 x 10 microl) were instilled three times in the left conjuctival sac at 40 min intervals. Pupil diameter was monitored with a binocular infrared television pupillometer before and for 4.5 h after ingestion of the capsule. The pupillary response to tyramine was expressed as the area under the pupil diameter x time curve (arbitrary units). A blood sample was taken before and 2 h after ingestion of the capsule, for the assay of platelet MAO-B activity, and plasma 3,4-dihydroxyphenylglycol (DHPG) concentration, an index of MAO-A activity. Platelet MAO activity was assayed radiochemically, using [14C]-phenylethylamine as substrate, and plasma DHPG by high performance liquid chromatography (h.p.l.c.). The results were analysed using analysis of variance with repeated measures, followed by Bonferroni's corrected t-test, using a significance criterion of P < 0.05.
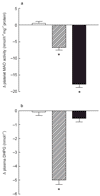
Results: Both moclobemide and selegiline, compared with placebo, caused significant miosis in the right (untreated) eye. The changes in pupil diameter (mm +/- s.e. mean) from the pretreatment measurement were: placebo -0.09 +/- 0.07, moclobemide -0.52 +/- 0.09, selegiline -0.26 +/- 0.1. The mydriatic response to tyramine was potentiated by moclobemide, compared with the response recorded in the presence of placebo. The responses to tyramine (arbitrary units +/- s.e. mean) were: placebo 77.08 +/- 11.65, moclobemide 140.25 +/- 18.9, selegiline 72.75 +/- 12.35. Both moclobemide and selegiline significantly reduced platelet MAO activity, compared with placebo. The changes in platelet MAO activity (nmol h(-1) mg(-1) protein +/- s.e. mean) from the pretreatment level were: placebo 0.5 +/- 0.62, moclobemide -6.7 +/- 0.66, selegiline -17.7 +/- 0.87. Moclobemide significantly reduced plasma DHPG concentration, compared with placebo. The changes in plasma DHPG concentration (nmol l(-1) +/- s.e. mean) from the pretreatment level were: placebo -0.01 +/- 0.24, moclobemide -4.98 +/- 0.32, selegiline -0.51 +/- 0.26.
Conclusions: The potentiation of tyramine-evoked mydriasis by moclobemide is likely to reflect the inhibition of MAO-A activity in the iris, consistent with the activity of this enzyme in sympathetic nerve terminals. The lack of effect of selegiline on tyramine-evoked mydriasis argues against a role of MAO-B in terminating the effects of sympathomimetic amines in the iris. The effects of the two drugs on platelet MAO activity and plasma DHPG concentration are in agreement with previous reports and consistent with the relative selectivity of moclobemide for MAO-A and of selegiline for MAO-B. The miosis caused by the two MAO inhibitors is likely to be due to a central sympatholytic action of the drugs.
Figures



Similar articles
-
Pharmacokinetic-pharmacodynamic interactions between two selective monoamine oxidase inhibitors: moclobemide and selegiline.Clin Neuropharmacol. 1996 Oct;19(5):399-414. doi: 10.1097/00002826-199619050-00003. Clin Neuropharmacol. 1996. PMID: 8889283 Clinical Trial.
-
Inhibition of monoamine oxidase by moclobemide: effects on monoamine metabolism and secretion of anterior pituitary hormones and cortisol in healthy volunteers.Br J Clin Pharmacol. 1989 Feb;27(2):243-55. doi: 10.1111/j.1365-2125.1989.tb05357.x. Br J Clin Pharmacol. 1989. PMID: 2469451 Free PMC article. Clinical Trial.
-
Relationship between tyramine potentiation and monoamine oxidase (MAO) inhibition: comparison between moclobemide and other MAO inhibitors.Acta Psychiatr Scand Suppl. 1990;360:81-3. doi: 10.1111/j.1600-0447.1990.tb05342.x. Acta Psychiatr Scand Suppl. 1990. PMID: 2248084
-
Therapeutic applications of selective and non-selective inhibitors of monoamine oxidase A and B that do not cause significant tyramine potentiation.Neurotoxicology. 2004 Jan;25(1-2):243-50. doi: 10.1016/S0161-813X(03)00103-7. Neurotoxicology. 2004. PMID: 14697899 Review.
-
Hypotensive action and weak potentiation of tyramine effect by moclobemide in rats.Acta Psychiatr Scand Suppl. 1990;360:106-7. doi: 10.1111/j.1600-0447.1990.tb05350.x. Acta Psychiatr Scand Suppl. 1990. PMID: 2248060 Review.
Cited by
-
Chronic Serotonergic Overstimulation Mimicking Panic Attacks in a Patient with Parkinson's Disease Receiving Additional Antidepressant Treatment with Moclobemide.Case Rep Psychiatry. 2021 Mar 1;2021:8868023. doi: 10.1155/2021/8868023. eCollection 2021. Case Rep Psychiatry. 2021. PMID: 33728085 Free PMC article.
-
Steady-state pharmacokinetics and pharmacodynamics of CHF3381, a novel antineuropathic pain agent, in healthy subjects.Br J Clin Pharmacol. 2005 Apr;59(4):405-14. doi: 10.1111/j.1365-2125.2005.02338.x. Br J Clin Pharmacol. 2005. PMID: 15801935 Free PMC article. Clinical Trial.
-
Human amygdala sensitivity to the pupil size of others.Cereb Cortex. 2008 Dec;18(12):2729-34. doi: 10.1093/cercor/bhn034. Epub 2008 Mar 27. Cereb Cortex. 2008. PMID: 18372291 Free PMC article.
References
-
- Berry MD, Juorio AV, Paterson IA. The functional role of monoamine oxidases A and B in the mammalian central nervous system. Progr Neurobiol. 1994;42:375–391. - PubMed
-
- Murphy DL, Sunderland T, Garrick NA, Aulakh CS, Cohen RM. Selective amine oxidase inhibitors: basic to clinical studies. In: Dahl SG, Gram LF, Paul SM, Potter WZ, editors. Clinical Pharmacology in Psychiatry. Berlin: Springer-Verlag; 1987. pp. 135–146.
-
- Broadley KJ. Autonomic pharmacology. London: Taylor & Francis; 1996.
-
- Szabadi E, Bradshaw CM. Affective disorders: 1. Antidepressants. In. In: King DJ, editor. Seminars in Clinical Psychopharmacology. London: Royal College of Psychiatrists/Gaskall; 1995. pp. 138–192.
-
- Haefely W, Burkard WP, Cesura A, et al. Pharmacology of moclobemide. Clin Neuropharmacol. 1993;16(Suppl 2):S8–S18. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

