Eotaxin and capping protein in experimental vasculopathy
- PMID: 9665468
- PMCID: PMC1852962
- DOI: 10.1016/S0002-9440(10)65548-4
Eotaxin and capping protein in experimental vasculopathy
Abstract
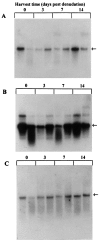
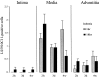
Ischemia-induced tissue activation may contribute to the pathogenesis of graft vasculopathy, but the mediators implicated have only partially been characterized. To gain further insight into the molecular mechanisms involved, syngeneic rat aortic transplants with cold-storage-induced vasculopathy were studied for differentially expressed mRNA transcripts. Vessel segments were exposed to either 1 or 18 hours of cold ischemia, followed by transplantation into syngeneic recipients. After 3 days or 4 weeks, the grafts were removed and total mRNA was isolated and used for differential display to identify modulation of transcript expression related to prolonged storage. Using 15 sets of random primers, 17 polymerase chain reaction products were up-regulated and 2 were downregulated in grafts exposed to 18 hours of ischemia. Sequencing of these amplicons showed that 6 had a high degree of homology to known sequences whereas 13 had no homology to any of the genes in the database. Two of the differentially displayed amplicons (capping protein and eotaxin) were cloned, re-amplified, and used as probes for Northern blot analysis to confirm their differential expression. Immunohistochemistry using monoclonal antibodies against capping protein-alpha and eotaxin confirmed that both proteins are expressed in the media of normal aortas and that there was an increased expression in vessels exposed to prolonged ischemia albeit that the increase at the protein level seemed less compared with changes in transcript expression. Northern blots with RNA from aortic allografts exposed to prolonged ischemic storage also showed increased levels of capping protein and eotaxin mRNA whereas there was a decrease in the relative amount of these transcripts in vessels exposed to balloon denudation, suggesting that the increase after prolonged ischemic exposure is not the result of a nonspecific response to injury. Based on the biological characteristics of capping protein and eotaxin it is conceivable that they play a pathogenetic role in ischemia-induced vessel wall remodeling. It remains to be established whether these genes or their products serve as target molecules for therapeutic interventions to prevent or treat cold-storage-induced graft vasculopathy.
Figures







Similar articles
-
Identification of differentially expressed genes in rat aortic allograft vasculopathy.Am J Pathol. 1996 Aug;149(2):597-611. Am J Pathol. 1996. PMID: 8701998 Free PMC article.
-
Selective neutralization of the chemokine TCA3 reduces the increased injury of partial versus whole liver transplants induced by cold preservation.Transplantation. 2006 Dec 15;82(11):1501-9. doi: 10.1097/01.tp.0000243167.11566.eb. Transplantation. 2006. PMID: 17164723
-
Interleukin (IL)-5 downregulates tumor necrosis factor (TNF)-induced eotaxin messenger RNA (mRNA) expression in eosinophils. Induction of eotaxin mRNA by TNF and IL-5 in eosinophils.Am J Respir Cell Mol Biol. 1999 Sep;21(3):303-10. doi: 10.1165/ajrcmb.21.3.3467. Am J Respir Cell Mol Biol. 1999. PMID: 10460747
-
CXC chemokine expression and synthesis in skeletal muscle during ischemia/reperfusion.J Vasc Surg. 2005 Aug;42(2):337-43. doi: 10.1016/j.jvs.2005.04.046. J Vasc Surg. 2005. PMID: 16102636
-
Effects of Cold Ischemia on Gene Expression: A Review and Commentary.Biopreserv Biobank. 2016 Dec;14(6):548-558. doi: 10.1089/bio.2016.0013. Epub 2016 Aug 23. Biopreserv Biobank. 2016. PMID: 27551929 Free PMC article. Review.
Cited by
-
The role of FGF2 in migration and tubulogenesis of endothelial progenitor cells in relation to pro-angiogenic growth factor production.Mol Cell Biochem. 2015 Dec;410(1-2):131-42. doi: 10.1007/s11010-015-2545-5. Epub 2015 Aug 28. Mol Cell Biochem. 2015. PMID: 26314253
-
Kinetic and thermodynamic studies reveal chemokine homologues CC11 and CC24 with an almost identical tertiary structure have different folding pathways.BMC Biophys. 2017 Sep 12;10:7. doi: 10.1186/s13628-017-0039-4. eCollection 2017. BMC Biophys. 2017. PMID: 28919974 Free PMC article.
-
PPARA Polymorphism Influences the Cardiovascular Benefit of Fenofibrate in Type 2 Diabetes: Findings From ACCORD-Lipid.Diabetes. 2020 Apr;69(4):771-783. doi: 10.2337/db19-0973. Epub 2020 Jan 23. Diabetes. 2020. PMID: 31974142 Free PMC article. Clinical Trial.
-
Multiple Signaling Pathways Contribute to the Thrombin-induced Secretory Phenotype in Vascular Smooth Muscle Cells.Korean J Physiol Pharmacol. 2015 Nov;19(6):549-55. doi: 10.4196/kjpp.2015.19.6.549. Epub 2015 Oct 16. Korean J Physiol Pharmacol. 2015. PMID: 26557022 Free PMC article.
References
-
- Suzuki J, Isobe M, Morishita R, Aoki M, Horie S, Okubo Y, Kaneda Y, Sawa Y, Matsuda H, Ogihara T, Sekiguchi M: Prevention of graft coronary arteriosclerosis by antisense kinase oligonucleotide. Nature Med 1997, 3:900-903 - PubMed
-
- Yilmaz S, Paavonen T, Häyry P: Chronic rejection of rat renal allografts. II. The impact of prolonged ischemia time on transplant histology. Transplantation 1992, 53:823-827 - PubMed
-
- Wanders A, Akyürek ML, Waltenberger J, Ren ZP, Stafberg C, Funa K, Larsson E, Fellström B: Ischemia-induced transplant arteriosclerosis in the rat. Arterioscler Thromb 1995, 15:145-155 - PubMed
-
- Ojo AO, Wolfe RA, Held PJ, Port FK, Schmouder RL: Delayed graft function: risk factors and implications for renal allograft survival. Transplantation 1997, 63:968-974 - PubMed
-
- Kelly KJ, Bonventre JV: Ischemia/reperfusion injury in transplantation. Tilney NL Strom TB Paul LC eds. Transplantation Biology: Cellular and Molecular Aspects. 1996, :257-274 Lippincott-Raven, Philadelphia
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

