Expression and localization of matrix metalloproteinase-12 in the aorta of cholesterol-fed rabbits: relationship to lesion development
- PMID: 9665471
- PMCID: PMC1852935
- DOI: 10.1016/s0002-9440(10)65551-4
Expression and localization of matrix metalloproteinase-12 in the aorta of cholesterol-fed rabbits: relationship to lesion development
Abstract
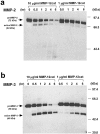
Degradation of extracellular matrix (ECM) proteins in the aorta is a critical step for the development of atherosclerosis. Expression of matrix metalloproteinase (MMP)-12 (macrophage elastase), an elastin-degrading proteinase in the MMP family, was investigated in the thoracic aorta of rabbits fed a 1% cholesterol-containing diet for 16 weeks. In the atherosclerotic lesions, MMP-12 was produced abundantly at both the mRNA and protein levels, whereas no expression was observed in the normal rabbit aortas. The principal source of MMP-12 was macrophage foam cells (MFCs) that had infiltrated the atherosclerotic intima; this was demonstrated in both in vitro culture studies of MFCs purified from atherosclerotic lesions and immunohistochemical studies of aortic lesions. Additional biochemical studies using recombinant rabbit MMP-12 revealed that MMP-12 digested elastin, type IV collagen, and fibronectin and also activated MMP-2 and MMP-3. Expression of MMP-12 by human macrophage cell lines was increased by stimulation with acetylated low-density lipoprotein, implying augmentation of MMP-12 production during foam cell formation. Increased expression of MMP-12 in atherosclerotic lesions, concomitant with foam cell generation, which triggers the acceleration of ECM breakdown, is likely to be a critical step in the initiation and progression of the atherosclerotic cascade.
Figures
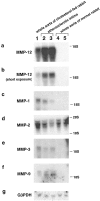


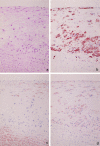
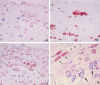

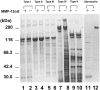

References
-
- Ross R: The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 1993, 362:801-809 - PubMed
-
- Watanabe T, Tokunaga O, Fan J, Shimokama T: Atherosclerosis and macrophages. Acta Pathol Jpn 1989, 39:473-486 - PubMed
-
- Dollery CM, McEwan JR, Henney AM: Matrix metalloproteinases and cardiovascular disease. Circ Res 1995, 77:863-868 - PubMed
-
- Holvoet P, Collen D: Oxidized lipoproteins in atherosclerosis and thrombosis. FASEB J 1994, 8:1279-1284 - PubMed
-
- Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL: Beyond cholesterol: modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med 1989, 320:915-924 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

