Development of a human gamma interferon enzyme immunoassay and comparison with tuberculin skin testing for detection of Mycobacterium tuberculosis infection
- PMID: 9665962
- PMCID: PMC95613
- DOI: 10.1128/CDLI.5.4.531-536.1998
Development of a human gamma interferon enzyme immunoassay and comparison with tuberculin skin testing for detection of Mycobacterium tuberculosis infection
Abstract
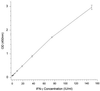
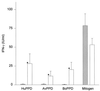
A sensitive two-step simultaneous enzyme immunoassay (EIA) for human gamma interferon (IFN-gamma) has been developed and used as an in vitro test for human tuberculosis (TB) in comparison with tuberculin skin testing. The EIA was shown to be highly sensitive, detecting less than 0.5 IU of recombinant human IFN-gamma per ml within a linear detection range of 0.5 to 150 IU/ml. The assay was highly reproducible and specific for native IFN-gamma. In addition, the assay detected chimpanzee, orangutan, gibbon, and squirrel monkey IFN-gammas. Cross-reactions with other human cytokines or with IFN-gammas derived from mice, cattle, or Old World monkeys were not evident. The assay was used to detect TB infection by incubating whole blood overnight with human, avian, and bovine tuberculin purified protein derivatives (PPDs), as well as positive (mitogen)- and negative-control preparations. The levels of IFN-gamma in plasma supernatants were then determined. Blood from 10 tuberculin skin test-positive individuals responded predominantly to the human tuberculin PPD antigen and to a lesser extent to bovine and avian PPD antigens. By contrast, blood from 10 skin test-negative individuals showed minimal responses or no response to any of the tuberculin PPDs. Detectable levels of IFN-gamma were present in all blood samples stimulated with mitogen. In vivo tuberculin reactivity was correlated with IFN-gamma responsiveness in vitro. These results support the further study of the blood culture-IFN-gamma EIA system as an alternative to skin testing for the detection of human TB infection.
Figures

References
-
- Al Orainey I O, Gad el Rab M O, al Hajjaj M S, Saeed E S. Detection of mycobacterial antigens in sputum by an enzyme immunoassay. Eur J Clin Microbiol Infect Dis. 1992;11:58–61. - PubMed
-
- Andersson G, Ekre H-P T, Alm G, Perlmann P. Monoclonal antibody two-site ELISA for human IFN-γ. Adaptation for determinations in human serum or plasma. J Immunol Methods. 1989;125:89–96. - PubMed
-
- Anonymous. Management, control and prevention of tuberculosis: guidelines for health care providers. Melbourne, Victoria, Australia: Victorian Government Department of Human Services Infectious Diseases Unit; 1996.
-
- Boerman O C, Segers M F G, Poels L G, Kenemans P, Thomas C M G. Heterophilic antibodies in human sera causing falsely increased results in the CA 125 immunofluorometric assay. Clin Chem. 1990;36:888–891. - PubMed
-
- Boscato L M, Stuart M C. Heterophilic antibodies: a problem for all immunoassays. Clin Chem. 1988;34:27–33. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

