Campylobacter species and Guillain-Barré syndrome
- PMID: 9665983
- PMCID: PMC88896
- DOI: 10.1128/CMR.11.3.555
Campylobacter species and Guillain-Barré syndrome
Abstract
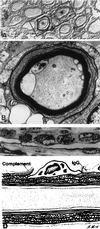
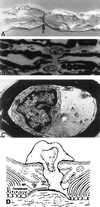
Since the eradication of polio in most parts of the world, Guillain-Barré syndrome (GBS) has become the most common cause of acute flaccid paralysis. GBS is an autoimmune disorder of the peripheral nervous system characterized by weakness, usually symmetrical, evolving over a period of several days or more. Since laboratories began to isolate Campylobacter species from stool specimens some 20 years ago, there have been many reports of GBS following Campylobacter infection. Only during the past few years has strong evidence supporting this association developed. Campylobacter infection is now known as the single most identifiable antecedent infection associated with the development of GBS. Campylobacter is thought to cause this autoimmune disease through a mechanism called molecular mimicry, whereby Campylobacter contains ganglioside-like epitopes in the lipopolysaccharide moiety that elicit autoantibodies reacting with peripheral nerve targets. Campylobacter is associated with several pathologic forms of GBS, including the demyelinating (acute inflammatory demyelinating polyneuropathy) and axonal (acute motor axonal neuropathy) forms. Different strains of Campylobacter as well as host factors likely play an important role in determining who develops GBS as well as the nerve targets for the host immune attack of peripheral nerves. The purpose of this review is to summarize our current knowledge about the clinical, epidemiological, pathogenetic, and laboratory aspects of campylobacter-associated GBS.
Figures


References
-
- Adams R D, Victor M. Diseases of the peripheral nerves. In: Adams R D, Victor M, editors. Principles of neurology. New York, N.Y: McGraw-Hill, Inc.; 1993. pp. 1117–1169.
-
- Apostolski S, Sadiq S A, Hays A, Corbo M, Suturkova-Milosevic L, Chaliff P, Stefansson K, LeBaron R G, Ruoslahti E, Hays A P, Latov N. Identification of Gal(β1-3)GalNAc bearing glycoproteins at the nodes of Ranvier in peripheral nerve. J Neurosci Res. 1994;38:134–141. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

