High-mobility group chromatin proteins 1 and 2 functionally interact with steroid hormone receptors to enhance their DNA binding in vitro and transcriptional activity in mammalian cells
- PMID: 9671457
- PMCID: PMC109033
- DOI: 10.1128/MCB.18.8.4471
High-mobility group chromatin proteins 1 and 2 functionally interact with steroid hormone receptors to enhance their DNA binding in vitro and transcriptional activity in mammalian cells
Abstract
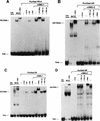
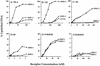
We previously reported that the chromatin high-mobility group protein 1 (HMG-1) enhances the sequence-specific DNA binding activity of progesterone receptor (PR) in vitro, thus providing the first evidence that HMG-1 may have a coregulatory role in steroid receptor-mediated gene transcription. Here we show that HMG-1 and the highly related HMG-2 stimulate DNA binding by other steroid receptors, including estrogen, androgen, and glucocorticoid receptors, but have no effect on DNA binding by several nonsteroid nuclear receptors, including retinoid acid receptor (RAR), retinoic X receptor (RXR), and vitamin D receptor (VDR). As highly purified recombinant full-length proteins, all steroid receptors tested exhibited weak binding affinity for their optimal palindromic hormone response elements (HREs), and the addition of purified HMG-1 or -2 substantially increased their affinity for HREs. Purified RAR, RXR, and VDR also exhibited little to no detectable binding to their cognate direct repeat HREs but, in contrast to results with steroid receptors, the addition of HMG-1 or HMG-2 had no stimulatory effect. Instead, the addition of purified RXR enhanced RAR and VDR DNA binding through a heterodimerization mechanism and HMG-1 or HMG-2 had no further effect on DNA binding by RXR-RAR or RXR-VDR heterodimers. HMG-1 and HMG-2 (HMG-1/-2) themselves do not bind to progesterone response elements, but in the presence of PR they were detected as part of an HMG-PR-DNA ternary complex. HMG-1/-2 can also interact transiently in vitro with PR in the absence of DNA; however, no direct protein interaction was detected with VDR. These results, taken together with the fact that PR can bend its target DNA and that HMG-1/-2 are non-sequence-specific DNA binding proteins that recognize DNA structure, suggest that HMG-1/-2 are recruited to the PR-DNA complex by the combined effect of transient protein interaction and DNA bending. In transient-transfection assays, coexpression of HMG-1 or HMG-2 increased PR-mediated transcription in mammalian cells by as much as 7- to 10-fold without altering the basal promoter activity of target reporter genes. This increase in PR-mediated gene activation by coexpression of HMG-1/-2 was observed in different cell types and with different target promoters, suggesting a generality to the functional interaction between HMG-1/-2 and PR in vivo. Cotransfection of HMG-1 also increased reporter gene activation mediated by other steroid receptors, including glucocorticoid and androgen receptors, but it had a minimal influence on VDR-dependent transcription in vivo. These results support the conclusion that HMG-1/-2 are coregulatory proteins that increase the DNA binding and transcriptional activity of the steroid hormone class of receptors but that do not functionally interact with certain nonsteroid classes of nuclear receptors.
Figures











References
-
- Adachi Y, Mizuno S, Yoshida M. Efficient large scale purification of non-histone chromosomal proteins HMG-1 and HMG-2 by using polybuffer exchanger PBE94. J Chromatogr. 1990;530:39–46. - PubMed
-
- Aizawa S, Nishino H, Saito K, Kimura K, Shirakawa H, Yoshida M. Stimulation of transcription in cultured cells by high mobility group protein 1: essential role of the acidic carboxyl-terminal region. Biochemistry. 1994;33:14690–14695. - PubMed
-
- Allegretto E A, McClurg M R, Lazarchik S B, Clemm D L, Kerner S A, Elgort M G, Boehm M F, White S K, Pike J W, Heyman R A. Transactivation properties of retinoic acid and retinoid X receptors in mammalian cells and yeast: correlation with hormone binding and effects of metabolism. J Biol Chem. 1993;268:26625–26633. - PubMed
-
- Allgood V E, Zhang Y, O’Malley B W, Weigel N L. Analysis of chicken progesterone receptor function and phosphorylation using an adenovirus mediated procedure for high efficiency DNA transfer. Biochemistry. 1997;36:224–232. - PubMed
-
- Burns L, Duggan B, Atkinson E A, Famulski K S, Nemer M, Bleackley R C, Michalak M. Modulation of gene expression by calreticulin binding to the glucocorticoid receptor. Nature. 1994;376:476–480. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
