Disruption of higher-order folding by core histone acetylation dramatically enhances transcription of nucleosomal arrays by RNA polymerase III
- PMID: 9671473
- PMCID: PMC109049
- DOI: 10.1128/MCB.18.8.4629
Disruption of higher-order folding by core histone acetylation dramatically enhances transcription of nucleosomal arrays by RNA polymerase III
Abstract
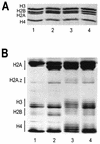
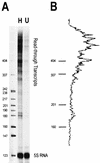
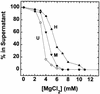
We have examined the effects of core histone acetylation on the transcriptional activity and higher-order folding of defined 12-mer nucleosomal arrays. Purified HeLa core histone octamers containing an average of 2, 6, or 12 acetates per octamer (8, 23, or 46% maximal site occupancy, respectively) were assembled onto a DNA template consisting of 12 tandem repeats of a 208-bp Lytechinus 5S rRNA gene fragment. Reconstituted nucleosomal arrays were transcribed in a Xenopus oocyte nuclear extract and analyzed by analytical hydrodynamic and electrophoretic approaches to determine the extent of array compaction. Results indicated that in buffer containing 5 mM free Mg2+ and 50 mM KCl, high levels of acetylation (12 acetates/octamer) completely inhibited higher-order folding and concurrently led to a 15-fold enhancement of transcription by RNA polymerase III. The molecular mechanisms underlying the acetylation effects on chromatin condensation were investigated by analyzing the ability of differentially acetylated nucleosomal arrays to fold and oligomerize. In MgCl2-containing buffer the folding of 12-mer nucleosomal arrays containing an average of two or six acetates per histone octamer was indistinguishable, while a level of 12 acetates per octamer completely disrupted the ability of nucleosomal arrays to form higher-order folded structures at all ionic conditions tested. In contrast, there was a linear relationship between the extent of histone octamer acetylation and the extent of disruption of Mg2+-dependent oligomerization. These results have yielded new insight into the molecular basis of acetylation effects on both transcription and higher-order compaction of nucleosomal arrays.
Figures



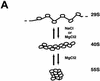
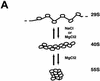
References
-
- Alland L, Muhle R, Hou H, Jr, Potes J, Chin L, Schreiber-Agus N, DePinho R A. Role for N-CoR and histone deacetylase in Sin3-mediated transcriptional repression. Nature. 1997;387:49–55. - PubMed
-
- Annunziato A T, Frado L L, Seale R L, Woodcock C L. Treatment with sodium butyrate inhibits the complete condensation of interphase chromatin. Chromosoma. 1988;96:132–138. - PubMed
-
- Ausio J, van Holde K E. Histone hyperacetylation: its effects on nucleosome conformation and stability. Biochemistry. 1986;25:1421–1428. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
