Regulation of differentiation by HBP1, a target of the retinoblastoma protein
- PMID: 9671483
- PMCID: PMC109059
- DOI: 10.1128/MCB.18.8.4732
Regulation of differentiation by HBP1, a target of the retinoblastoma protein
Abstract
Differentiation is a coordinated process of irreversible cell cycle exit and tissue-specific gene expression. To probe the functions of the retinoblastoma protein (RB) family in cell differentiation, we isolated HBP1 as a specific target of RB and p130. Our previous work showed that HBP1 was a transcriptional repressor and a cell cycle inhibitor. The induction of HBP1, RB, and p130 upon differentiation in the muscle C2C12 cells suggested a coordinated role. Here we report that the expression of HBP1 unexpectedly blocked muscle cell differentiation without interfering with cell cycle exit. Moreover, the expression of MyoD and myogenin, but not Myf5, was inhibited in HBP1-expressing cells. HBP1 inhibited transcriptional activation by the MyoD family members. The inhibition of MyoD family function by HBP1 required binding to RB and/or p130. Since Myf5 might function upstream of MyoD, our data suggested that HBP1 probably blocked differentiation by disrupting Myf5 function, thus preventing expression of MyoD and myogenin. Consistent with this, the expression of each MyoD family member could reverse the inhibition of differentiation by HBP1. Further investigation implicated the relative ratio of RB to HBP1 as a determinant of whether cell cycle exit or full differentiation occurred. At a low RB/HBP1 ratio cell cycle exit occurred but there was no tissue-specific gene expression. At elevated RB/HBP1 ratios full differentiation occurred. Similar changes in the RB/HBP1 ratio have been observed in normal C2 differentiation. Thus, we postulate that the relative ratio of RB to HBP1 may be one signal for activation of the MyoD family. We propose a model in which a checkpoint of positive and negative regulation may coordinate cell cycle exit with MyoD family activation to give fidelity and progression in differentiation.
Figures

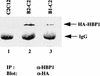


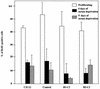
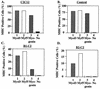

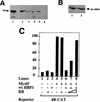

References
-
- Barone M V, Crozat A, Tabaee A, Philipson L, Ron D. CHOP (GADD153) and its oncogenic variant, TLS-CHOP, have opposing effects on the induction of G1/S arrest. Genes Dev. 1994;8:453–464. - PubMed
-
- Braun T, Rudnicki M, Arnold H-H, Jaenisch R. Targeted inactivation of the muscle regulatory gene Myf-5 results in abnormal rib development and perinatal death. Cell. 1992;71:369–382. - PubMed
-
- Chen P L, Riley D J, Chen Y, Lee W H. Retinoblastoma protein positively regulates terminal adipocyte differentiation through direct interaction with C/EBPs. Genes Dev. 1996;10:2794–2804. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
