Cytoplasmic tail regulates the intercellular adhesion function of the epithelial cell adhesion molecule
- PMID: 9671492
- PMCID: PMC109068
- DOI: 10.1128/MCB.18.8.4833
Cytoplasmic tail regulates the intercellular adhesion function of the epithelial cell adhesion molecule
Abstract
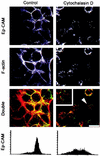
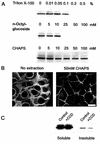
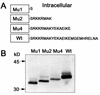
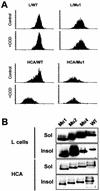
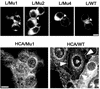
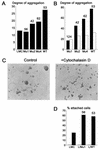
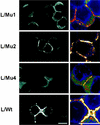
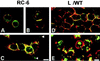
Ep-CAM, an epithelium-specific cell-cell adhesion molecule (CAM) not structurally related to the major families of CAMs, contains a cytoplasmic domain of 26 amino acids. The chemical disruption of the actin microfilaments, but not of the microtubuli or intermediate filaments, affected the localization of Ep-CAM at the cell-cell boundaries, suggesting that the molecule interacts with the actin-based cytoskeleton. Mutated forms of Ep-CAM were generated with the cytoplasmic domain truncated at various lengths. All of the mutants were transported to the cell surface in the transfectants; however, the mutant lacking the complete cytoplasmic domain was not able to localize to the cell-cell boundaries, in contrast to mutants with partial deletions. Both the disruption of the actin microfilaments and a complete truncation of the cytoplasmic tail strongly affected the ability of Ep-CAM to mediate aggregation of L cells. The capability of direct aggregation was reduced for the partially truncated mutants but remained cytochalasin D sensitive. The tail truncation did not affect the ability of the transfectants to adhere to solid-phase-adsorbed Ep-CAM, suggesting that the ability to form stable adhesions and not the ligand specificity of the molecule was affected by the truncation. The formation of intercellular adhesions mediated by Ep-CAM induced a redistribution to the cell-cell boundaries of alpha-actinin, but not of vinculin, talin, filamin, spectrin, or catenins. Coprecipitation demonstrated direct association of Ep-CAM with alpha-actinin. Binding of alpha-actinin to purified mutated and wild-type Ep-CAMs and to peptides representing different domains of the cytoplasmic tail of Ep-CAM demonstrates two binding sites for alpha-actinin at positions 289 to 296 and 304 to 314 of the amino acid sequence. The results demonstrate that the cytoplasmic domain of Ep-CAM regulates the adhesion function of the molecule through interaction with the actin cytoskeleton via alpha-actinin.
Figures



References
-
- Ben-Ze’ev A. Cytoskeletal and adhesion proteins as tumor suppressors. Curr Opin Cell Biol. 1997;9:99–108. - PubMed
-
- Bergsagel P L, Victor-Kobrin C, Timblin C R, Trepel J, Kuehl W L. A murine cDNA encodes a pan-epithelial glycoprotein that is also expressed on plasma cells. J Immunol. 1992;148:590–596. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
