Structure-function analysis of Qk1: a lethal point mutation in mouse quaking prevents homodimerization
- PMID: 9671495
- PMCID: PMC109071
- DOI: 10.1128/MCB.18.8.4863
Structure-function analysis of Qk1: a lethal point mutation in mouse quaking prevents homodimerization
Abstract
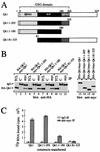
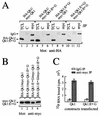
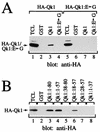
Qk1 is a member of the KH domain family of proteins that includes Sam68, GRP33, GLD-1, SF1, and Who/How. These family members are RNA binding proteins that contain an extended KH domain embedded in a larger domain called the GSG (for GRP33-Sam68-GLD-1) domain. An ethylnitrosourea-induced point mutation in the Qk1 GSG domain alters glutamic acid 48 to a glycine and is known to be embryonically lethal in mice. The function of Qk1 and the GSG domain as well as the reason for the lethality are unknown. Here we demonstrate that the Qk1 GSG domain mediates RNA binding and Qk1 self-association. By using in situ chemical cross-linking studies, we showed that the Qk1 proteins exist as homodimers in vivo. The Qk1 self-association region was mapped to amino acids 18 to 57, a region predicted to form coiled coils. Alteration of glutamic acid 48 to glycine (EG) in the Qk1 GSG domain (producing protein Qk1:EG) abolishes self-association but has no effect on the RNA binding activity. The expression of Qk1 or Qk1:EG in NIH 3T3 cells induces cell death by apoptosis. Approximately 90% of the remaining transfected cells are apoptotic 48 h after transfection. Qk1:EG was consistently more potent at inducing apoptosis than was wild-type Qk1. These results suggest that the mouse quaking lethality (EG) occurs due to the absence of Qk1 self-association mediated by the GSG domain.
Figures

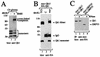


References
-
- Baehrecke E H. who encodes a KH RNA binding protein that functions in muscle development. Development. 1997;124:1323–1332. - PubMed
-
- Barbarese E. Spatial distribution of myelin basic protein mRNA and polypeptide in Quaking oligodendrocytes in culture. J Neurosci Res. 1991;29:271–281. - PubMed
-
- Bartoszewicz Z P, Noronha A B, Fujita N, Sato S, Bo L, Trapp B D, Quarles R H. Abnormal expression and glycosylation of the large and small isoforms of myelin-associated glycoprotein in dysmyelinating quaking mutants. J Neurochem Res. 1995;41:27–38. - PubMed
-
- Benda P, Lightbody J, Sato G, Levine L, Sweet W. Differentiated rat glial cell strain in tissue culture. Science. 1968;161:370–371. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
